Competencies and Facilities
Our group excels in state-of-the-art research infrastructure and analytical expertise. Whether you are a student seeking searching for project opportunities or a researcher exploring collaborations, explore our key competencies below - let us find synergy and drive innovation within plant sciences together!

Oxygen microsensors
We use O₂ microsensors to measure tissue O₂ status or calculate O₂ fluxes from diffusion gradients. With their tiny tips, O₂ microsensors offer excellent spatial resolution, and the automated microstage can position the sensor tip within ±1 µm. The microsensor is a miniaturized Clark-type O₂ sensor, capable of measuring O₂ concentration (or more precisely, O₂ partial pressure) with an accuracy of 0.1 µmol O₂ L⁻¹. We can assess tissue O₂ status in both submerged and aerial tissues of roots, stems, leaves, and fruits. A recent and novel application of these O₂ (or H₂S) microsensors is measuring the permeance of these gases across discrete tissue layers. All our microsensor equipment is based on the state-of-art platform provided by Unisense.
Other microsensors
In addition to molecular O₂, we can also measure molecular hydrogen sulfide (H₂S), hydrogen (H₂), temperature, and pH with extremely high spatial resolution inside plant tissues. If you believe these technologies fit your application, please do not hesitate to contact us to explore potential collaboration.

Micro Respiration
Our Unisense MicroResp equipment is frequently used to assess tissue O₂ consumption in root or shoot segments. It provides reliable O₂ consumption rates for tissue samples as small as 10 mg fresh mass, allowing us to resolve gradients in O₂ consumption along a root. The system can process up to eight samples simultaneously, each incubated in a micro-cuvette with a volume ranging from 0.5 to 4 mL. An optical O₂ sensor moves between cuvettes, and the software calculates O₂ consumption between two time points.

Radial O2 loss
We can measure radial O₂ loss from roots using three very different approaches. Two of these methods provide detailed information on O₂ efflux along the root axis, while the third estimates total O₂ loss from the entire root system to an anoxic medium. All three methods have scientific merit, and the choice depends on the specific research question. The method that estimates whole-root O₂ loss is particularly suitable for screening large numbers of genotypes. However, all three methods share the requirement that plants must be cultivated in hydroponics.
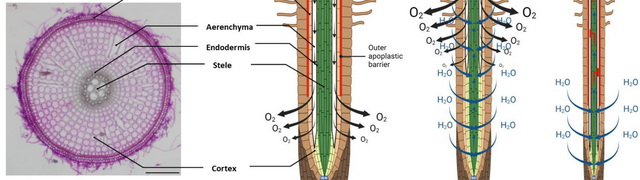 Root anatomy
Root anatomy
We have a state-of-the-art Leica VT1200-S vibrating microtome, enabling us to produce high-quality cross-sections of fresh root material. Additionally, we employ histological staining techniques to visualize cell wall components such as lignin and suberin. These cross-sections are then examined using our Nikon Ci-L microscope, which is equipped with LED fluorescence and the relevant excitation and emission filters.
Root architecture
Root architecture is a key trait that responds to abiotic stress but is inherently difficult to assess accurately. However, with our Epson Expression 13000XL Pro, we can scan root systems at up to 1200 × 4800 DPI and process these high-resolution images using RhizoVision. This allows us to classify roots into different categories, calculate root surface area, and perform various other analyses (see here for examples).
Leaf gas films
Leaf hydrophobicity, or leaf gas film formation, is a key trait that contributes to submergence tolerance in many wetland plants, including rice. We have specialized equipment to measure gas film thickness, typically ranging from 5 to 30 µm, using a buoyancy-based approach. Check out this YouTube video, where we explain the measurement process and the required equipment.
Underwater net photosynthesis
Underwater net photosynthesis in submerged plants, including terrestrial species, supplies carbohydrates and O₂ during submergence, making it a critical trait for survival during flood events. We use a vertically rotating incubator with full-spectrum daylight to measure net photosynthesis rates in leaf segments incubated in glass vials under ambient conditions or elevated CO₂ or light. The approach relies on O₂ evolution during the incubation, which is measured with an optical O₂ sensor. Check out this YouTube video if you are curious to see how it works!
Photosynthesis in air
Our group has a brand-new LI-6800 photosynthesis IRGA, which is used to measure net photosynthesis in air. Unlike underwater net photosynthesis, which is based on O₂ evolution, the IRGA measures CO₂ uptake or production. The LI-6800 is portable and suitable for field measurements; however, we primarily use it in our constant-temperature rooms or glasshouse.

Our Li-Cor CO₂ and CH₄ analyzer is one of our most frequently used instruments. We utilize it to assess plant-mediated greenhouse gas fluxes in custom-built flux chambers designed to accommodate our standard pots for both hydroponic and soil-based rice cultures. Additionally, we have developed a system that enables this device to measure soil CO₂ and CH₄ concentrations - an analysis typically conducted using gas chromatography.
Tissue Na and K In our salinity tolerance studies, we use a Jenway FPP7 Clinical Flame Photometer for tissue analyses of sodium and potasium.
Tissue Cl Similarly to tissue Na and K, tissue chloride is an important analysis in studies of salinity tolerance. We use a Genotec chloridometer for these analyses.
Osmotic pressure Tissue osmotic pressue can easily be measured with our osmometer model 3250 from Advanced Instruments.
Pigment analysis Chlorophylls and other pigements are measured spectrophotometrically with our Shimadzu UV-1800 spectrophotometer.
Greenhouse gases We have a brand-new Shimadzu gas chromatograph (model 51586) equipped with a headspace unit, making it ideal for the analysis of CO₂, N₂O, and CH₄ in both aqueous and gas samples.
Sugars and starch We have developed a new protocol for measuring both sugars and starch in very small tissue samples — down to 1 mg dry mass.
Contact
Professor Ole Pedersen
Freshwater Biology
Universitetsparken 4, 3rd floor
DK-2100 Copenhagen
opedersen@bio.ku.dk
Mobile: +45 23747641
ORCID: 0000-0002-0827-946X
Publications

Publications by Ole Pedersen are accessible from here!
Student projects

Are you curious to see what you can do during your BSc or MSc thesis project? Check it out on this link!
Video guides
 Click here to access our quick guide video library
Click here to access our quick guide video library