DARWIN - Dynamics of Antimicrobial Resistance in the urban Water Cycle in Europe
In DARWIN, we will undertake a never-previously-performed pan-European examination of the fate of key AMR organisms and genetic determinants in Urban Water Systems (UWS) resulting from discharged hospital and community wastes, including transmission mechanisms in different stages of sewer catchments and receiving waters.
More detailed text about the research being carried out in no more than 800 characters. Include the most important search words in this section. More detailed text about the research being carried out in no more than 800 characters. Include the most important search words in this section.
More detailed text about the research being carried out in no more than 800 characters. Include the most important search words in this section.
DARWIN - Dynamics of Antimicrobial Resistance in the urban Water Cycle in Europe
In DARWIN, we will undertake a never-previously-performed pan-European examination of the fate of key AMR organisms and genetic determinants in Urban Water Systems (UWS) resulting from discharged hospital and community wastes, including transmission mechanisms in different stages of sewer catchments and receiving waters.
Background and aim
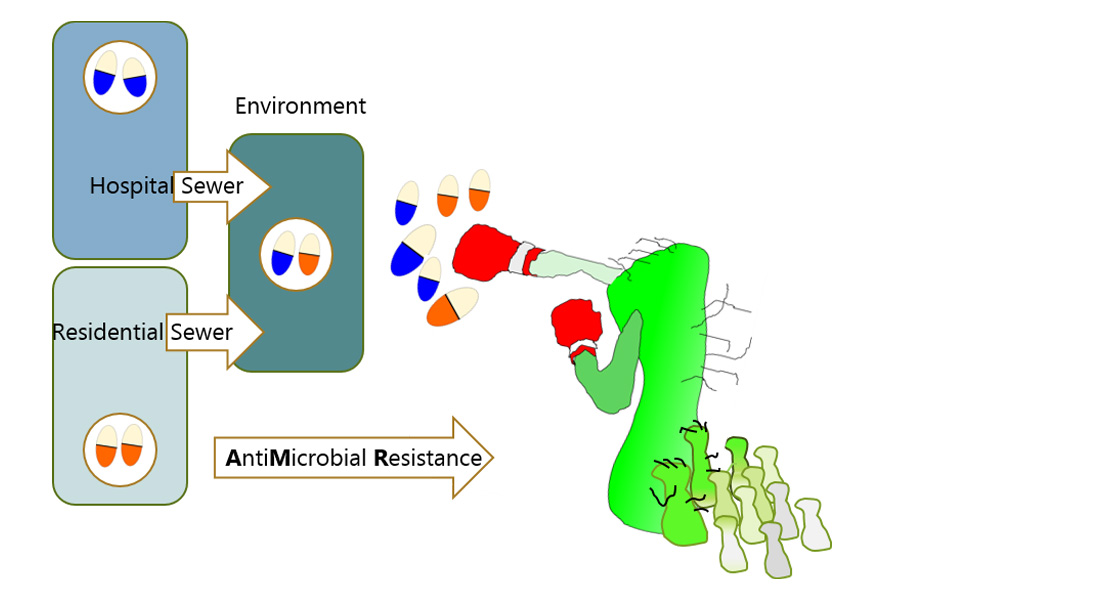
Resistance of pathogenic bacteria to antibiotics and other antimicrobials is increasing everywhere on Earth. This is making progressively our current antibiotics useless, potentially placing patient's lives at risk, as some can no longer be treated by available treatment options. As AntiMicrobial Resistance (AMR) continues to expand new AMR mitigation approaches are urgently needed that go beyond just prudent antimicrobial use. Specifically, AMR will not be reduced until we understand all key drivers of AMR transmission, including human waste-born AMR disseminated via urban water systems. Urban AMR dissemination starts when one flushes the toilet and sewage flows into the sewer system.
AMR organisms and genes are enriched in the gut due to antimicrobial use and natural selection, but when gut bacteria enter the environment with faeces they are exposed to different environmental conditions with changes in temperature, richness of organic matter, redox conditions, and levels of antibiotics, metals, and biocides. While enteric bacteria tend to quickly die off in UWSs, this does not prevent dissemination of AMR genes to environmental strains with whom they comingle. Comingling is especially concerning where hospital and community wastes mix, such as in sewers. This mixing creates a major opportunity for Horizontal gene transfer (HGT) between faecal and non-faecal strains, and fuels the passage of AMR genes via environmental strains to the wider environment.
In this project, we will perform a cross-European examination of the fate of key resistant bacteria and resistance genes in UWSs resulting from discharged hospital and community wastes, including mechanisms of resistance gene transfer in different stages of sewer catchments and receiving waters. We will focus on the spread of resistance genes that are amongst the most problematic now because they confer resistance to the latest generation of antibiotics available (genes for extended spectrum beta-lactamases (ESBL) and carbapenemases). We postulate that resistance genes readily transmit in urban water systems from pathogenic and non-pathogenic "gut" bacteria in human wastes (after antibiotic use) to environmental bacteria better adapted to the sewer environment. The environmental bacteria then carry the resistance genes across the wider environment, increasing community exposure. 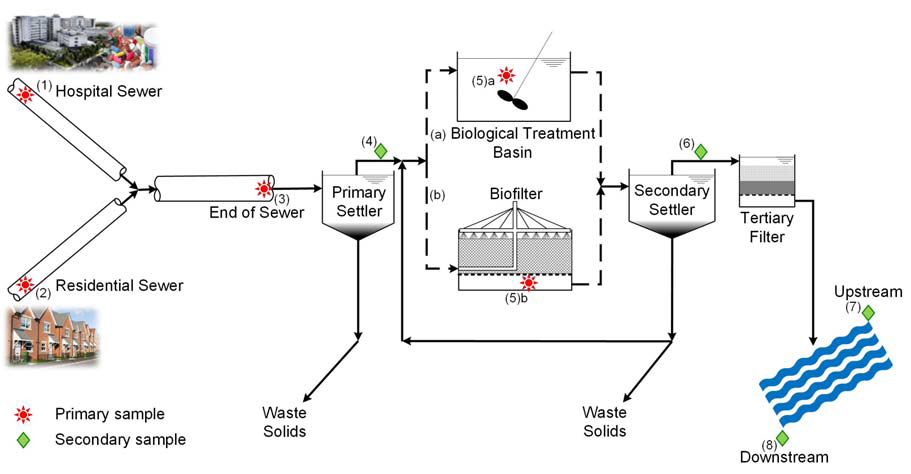 Hence, we will, for the first time, determine which specific bacteria carry the resistance genes across the urban water systems and identify where resistance gene transfer events occur. Our ultimate goal is to assess the relative risk of resistance genes returning back to humans due to environmental exposure.
Hence, we will, for the first time, determine which specific bacteria carry the resistance genes across the urban water systems and identify where resistance gene transfer events occur. Our ultimate goal is to assess the relative risk of resistance genes returning back to humans due to environmental exposure.
Perspectives
The DARWIN team will by employing novel tools and methods evaluate bacteria that perform HGT within UWSs, and thereby identify ecosystem controls on AMR transmission, ultimately developing a predictive dynamic AMR model to guide risk assessments and sewage management decisions. This will have significant implications on monitoring resistance and minimize the release or introduction of AMR in the environment as well as the human exposure to them.
Follow the link to read more about DARWIN
Consortium Coordinator
Professor Barth Smet
Technical University of Denmark
Department of Environmental Engineering
Partners
Technical University of Denmark
Department of Environmental Engineering
Newcastle University
School of Civil Engineering and Geoscienses
University of Birmingham
Centre for Computational Biology
Universidade de Santiago de Compostela
Department of Microbiology and Parasitology
University Hospital Complex of Santiago de Compostela
Instituto de Investigacíon
Rambam Health Care Campus
Division of Infectious Disease, Rambam Health Care Campus
& Bruce Rappaport School of Medicine
 Professor Søren Johannes Sørensen
Professor Søren Johannes Sørensen
I am currently team leader for a group of 12 scientists, 11 phd’s, three technicians and ten master students applying molecular techniques in microbial ecological studies at the Section of Microbiology. My research focuses on social interactions in microbial populations. My group’s studies evaluate the extent of genetic flow within natural communities and their response to environmental perturbations.
The section houses state of the art culture independent experimental infrastructure that enables us to examine how microbes interact at a scale that is relevant for such small organisms and to examine and identify the roles of the individual species that contribute to these interactions.
We investigate how microbes work together in in their natural state such as biofilms and the effect this has on microbial communities in in vivo systems such as human or animal gut, soil, or phase change communities. These are related to our in vitro model systems such as our bioflux biofilm system where we can evaluate our findings and examine phenomenon such as horizontal gene transfer or emergent community properties and evolutionary strategies. To do so we use techniques such as high throughput sequencing, flow cytometry and ultra-high resolution bioimaging or confocal microscopy and microbial reporter systems.
The pioneering work of the section has had been recognized for its impact in diverse areas in which microbial interactions are critical. These include, Soil biodiversity and bioremediation, the role of horizontal gene transfer, human disease progression, the effects of species diversity in plant animal and environmental microbiomes, how microbes interact in evolutionary strategies and the significance of cooperation verses antagonism in microbial systems.
Working in these areas necessitates close collaboration with international scientists from around the world that come to the section and to whom members of the section visit in scientific exchange programs. They span all corners of the globe including Dr. Duncan Veal, Macquarie University, Sydney Australia, Prof A. Spormann, Stanford University, Prof. S. Kjelleberg, University of NSW; Prof. M. Schloter, Helmholtz Zentrum München; and Prof. Md Hans Bisgaard COPSAC back here in Denmark.
These collaborations have helped more than 20 postdocs and 30 past Ph.D. fellows follow their career path both here and around the world to obtain successful positions in both academia and research.
Section of Microbiology

Funded by:
Project title: DARWIN - Dynamics of Antimicrobial Resistance in the urban Water Cycle in Europe
Project period: 01.01.2017 - 31.12.2019
Contact
 Professor
Professor
Søren J. Sørensen
Universitetsparken 15
Building 1, 1st floor
DK-2100 Copenhagen
Email: sjs@bio.ku.dk


