Embedding bacteria in implants to prevent infection - BioEmbed
The aim of BioEmbed is to develop and test a novel approach for embedding live probiotic bacteria into the catheter material and, thereby, reduce the attachment of pathogenic bacteria
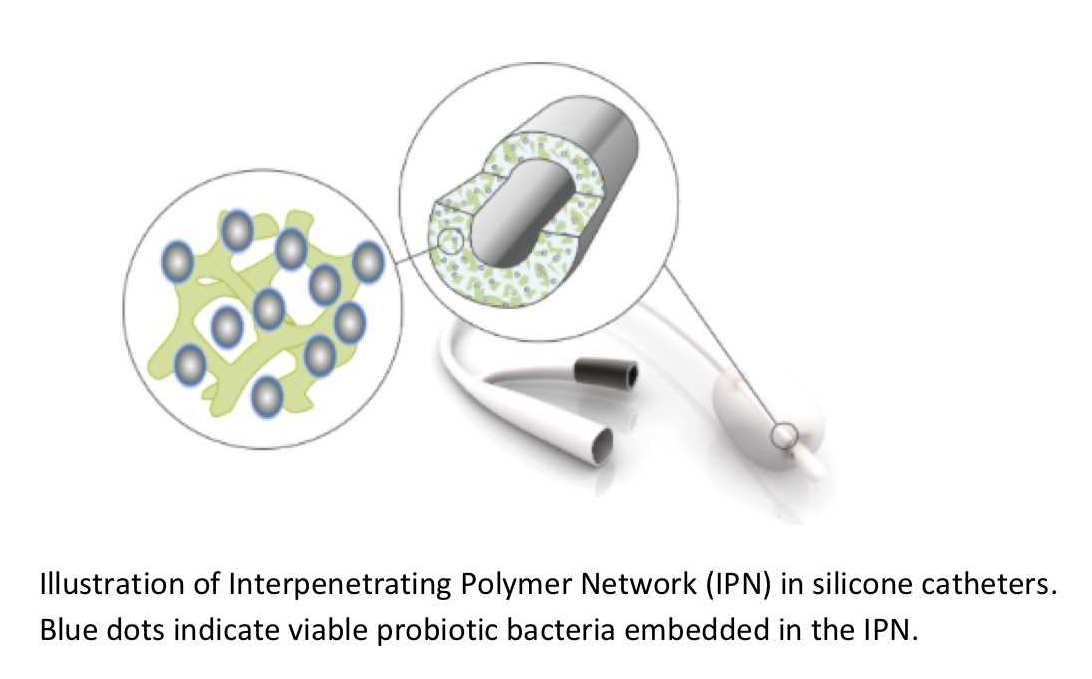
Bacterial colonisation of indwelling devices, such as catheters and stents, is often the cause of severe infections, including catheter-associated urinary tract infections (CAUTI), urinary tract and renal infections and, in severe cases, bacteraemia. These infections can be hard to eradicate, as the attached bacteria tolerate high doses of antibiotics, and in some cases, the infection persists even after device removal.
The aim of BioEmbed is to develop and test a novel approach for embedding live probiotic bacteria into the catheter material and, thereby, reduce the attachment of pathogenic bacteria.
 Embedding bacteria in implants to prevent infection - BioEmbed
Embedding bacteria in implants to prevent infection - BioEmbed
The use of indwelling devices confers a high risk of associated infectious diseases. These are caused by multiple bacterial species colonising the device, resulting in bacterial biofilm formation that is highly tolerant to antibiotics and hard to eradicate. Despite attempts to reduce biofilm formation using catheter surface coatings, no long-term solutions have yet been identified. With BioEmbed, I propose to apply a novel method for preventing device associated infection: I suggest embedding live probiotic bacteria into the device to prevent pathogen attachment and growth. This fundamentally new approach will challenge the medical dogma of antimicrobial induced sterility whilst reducing infections.
In the project, I will collaborate with a Danish biotech company to develop and evaluate a methodology for embedding probiotic bacteria in urinary catheters. The inhibitory effect of the embedded bacteria on pathogens will be assessed using bacterial isolates from catheter infections.
The impact and next step
BioEmbed will develop and evaluate a methodology that will enable improved, bioactive devices and novel uses of probiotics, ultimately reducing the prevalence of CAUTI. When extended to other devices, the safe bacterial entrapment allows the use of embedded devices at body sites not previously identified as targets for use of probiotics. This may become a crucial turning point in probiotic application, challenging traditional infection prevention and treatment regimes that aim at sterility. In comparison to traditional methods, this novel strategy is sustainable, as it does not depend on antimicrobial therapy with itsunwanted selection for development of antibiotic resistant infections.
 My main research interest is the interplay and interactions among bacteria of different species within diverse communities. It is becoming more and more clear that the activity of bacteria are highly dependent on and determined by the microbial community they live in, and that the total functions of a multispecies bacterial community can’t be explained by examining each part of the community in isolation. Thus, a deeper exploration of the interactions shaping these communities is vital for understanding bacterial activity, physiology, function and evolution.
My main research interest is the interplay and interactions among bacteria of different species within diverse communities. It is becoming more and more clear that the activity of bacteria are highly dependent on and determined by the microbial community they live in, and that the total functions of a multispecies bacterial community can’t be explained by examining each part of the community in isolation. Thus, a deeper exploration of the interactions shaping these communities is vital for understanding bacterial activity, physiology, function and evolution.
My current research aims at understanding the prevalence and underlying mechanism of interspecific interactions; both with respect to interaction characterization (synergism vs. antagonism), main facilitator (co-metabolism, co-aggregation etc.) and molecular mechanism. This includes characterisation of the impact of quorum sensing and horizontal gene transfer. I study bacterial interactions in strain collections from various natural and human health-related environments including soil, marine, freshwater, day care facilities and food processing environments.
In most cases, tight bacterial associations in a structured community are prerequisites for the interactions to develop; therefore biofilms are perfect settings for studies of interspecific bacterial interactions. I my group, different types of biofilm models, including static, batch biofilms and the newly established biofilm flow system, Bioflux 1000z, have become our model systems of choice for study of multispecies interactions.
Section of Microbiology

Funded by:
Lundbeck Experiment Grant from the Lundbeck Foundation
Project: Embedding bacteria in implants to prevent infection - BioEmbed
Period: 01.06.2020 - 31.05.2022
Contact
 Associate professor
Associate professor
Mette Burmølle
Universitetsparken 15
Building 1, 1st floor
DK-2100 Copenhagen
Email: burmolle@bio.ku.dk

