Modeling Asthma Development
The aim of this project is to create an in vitro model system for investigating the host-microbiome interaction in the lungs that modulate risk of developing asthma later in life.
Asthma is becoming a burden worldwide, with +350 million estimated suffers and rising
prevalence. The risk of asthma is dependent on both maternal asthma status and bacterial exposure. Early life fecal microbiome has been linked to later development of asthma and other inflammatory disorders. Studies have similarly linked airway microbiome to health-disease transition, i.e. high diversity in early airway microbiome reduces asthma risk
and high abundance of specific bacteria increases asthma risk.
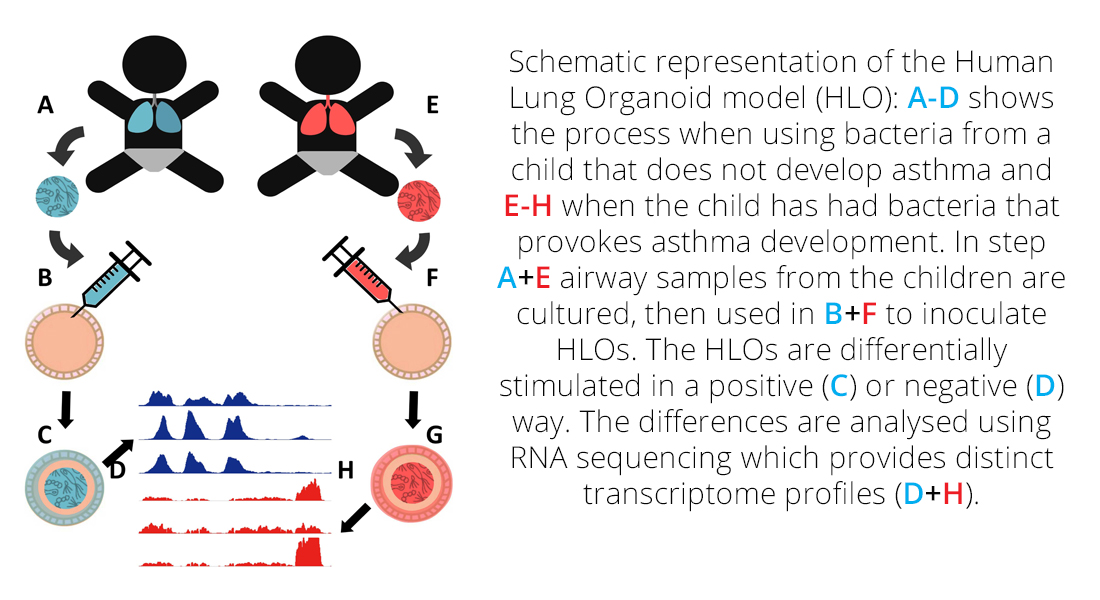
So far, no good model system for asthma exists, as asthma does not naturally occur in standard laboratory animals heritability cannot be studied, and with immune system differences to humans, the validity of using murine models to study modulation of later asthma development is questionable.
The aim of this project is to develop an Human Lung Organoid (HLO) model system for studying host-microbiome interactions modulating later risk of asthma, and determine the specific mechanisms involved, thus providing a leap forward in the understanding of asthma development.
Modeling Asthma Development
The prevalence of asthma has been rapidly increasing over the last half-century, at a rate that cannot be explained by human genetic mutations. The accepted hypothesis is that the early microbiome interacts with a child’s genetic makeup and that inappropriate microbiome composition can affect host immune maturation, thereby initiating a trajectory to immune-mediated diseases such as asthma. Furthermore, evidence suggests that asthma is the “canary in the coal mine”; the earliest and most common immune disorder debuting in the first years of life and a marker of more infrequent immune disorders.
Current studies are mostly observational, and the latest research has found that maternal asthma affects how early bacterial colonization changes the risk of developing asthma later in life. Some experimental studies have used murine models that can confirm some hypotheses but not identify causal mechanisms involving heritability, as mice do not naturally suffer from asthma.
We will combine knowledge of correlations between maternal genetics, microbiota, and asthma risk, with state-of-the-art human induced pluripotent stem cells (hPSCs) to develop a human lung organoid (HLO) model of the host cellular response to bacteria and subsequent asthma risk modulation. With such a model system we will finally be able to study the underlying causal mechanisms that shape the host-microbiome interactions
(HMI).
This project will develop an HLO model system for studying host-microbiome interactions modulating later risk of asthma, and determine the specific mechanisms involved, thus providing a leap forward in the understanding of asthma development.
Dr Trevor Lawley
Faculty Lead at the Host-Microbiota Interactions Lab (HMIL)
Wellcome Sanger Institute (WSI)
Cambridge, UK
 My research priorities focus how human health is affected by the microbiome, especially disease development. My primary focus has been investigating correlations between the microbiome and asthma development. I have a deep desire to understand the interaction mechanisms involved and how they affect human physiology and disease progression.
My research priorities focus how human health is affected by the microbiome, especially disease development. My primary focus has been investigating correlations between the microbiome and asthma development. I have a deep desire to understand the interaction mechanisms involved and how they affect human physiology and disease progression.
Section of Microbiology

Funded by:
Project name has received a three year funding from LINK
Project title: Modeling Asthma Development
Project period: 01.01.2019 - 30.06.2021
Contact
 Postdoc
Postdoc
Martin Steen Mortensen
Universitetsparken 15
Building 1, 1st floor
DK-2100 Copenhagen
Email: martin.mortensen@bio.ku.dk
and
The Host-Microbiota Interactions Lab (HMIL)
Wellcome Sanger Institute (WSI)
Cambridge
UK

