The role of low-virulence bacteria located on prosthetic breast implants in the development of capsular contracture
In this project we will investigate the role of a microbial community located on the surface of prosthetic breast implants in the development of a host immune reaction to the implant called capsular contracture.

This study aims to investigate the role of bacteria in the development of capsular contracture using Next Generation Sequencing (NGS) analysis and fluorescent in-situ hybridization (FISH) on prosthetic breast implants collected from patients who have their breast implants removed.
The role of low-virulence bacteria located on prosthetic breast implants in the development of capsular contracture
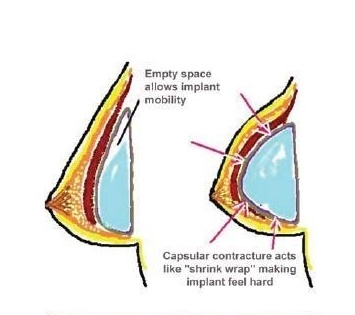 Capsular contracture is a common and debilitating complication to breast reconstruction with prosthetic implants where the hosts immune system rejects the implant by forming a thick fibrous capsule that contracts around the implant. We wish to characterize the microbial community (microbiome) located on the surface of the removed implants to identify the role of low-virulence bacteria in the development of inflammatory processes such as capsular contracture. We focus on a unique group of patients who have developed unilateral capsular contracture after a bilateral breast augmentation where both implants are available for testing. We hope to derive key insights into the mechanism that determines whether the immune system rejects or accepts a prosthetic implant.
Capsular contracture is a common and debilitating complication to breast reconstruction with prosthetic implants where the hosts immune system rejects the implant by forming a thick fibrous capsule that contracts around the implant. We wish to characterize the microbial community (microbiome) located on the surface of the removed implants to identify the role of low-virulence bacteria in the development of inflammatory processes such as capsular contracture. We focus on a unique group of patients who have developed unilateral capsular contracture after a bilateral breast augmentation where both implants are available for testing. We hope to derive key insights into the mechanism that determines whether the immune system rejects or accepts a prosthetic implant.
Gains and perspectives
This project has the prospect of identifying the key mechanisms responsible for the most common complication to breast reconstruction with implants affecting hundreds of patients each year in Denmark alone. Furthermore, by use of a patient group with unilateral capsular contracture, we will be able to isolate otherwise hidden differences in the complex microbial community found on the surface of the implants. This unique perspective has the potential to uncover universally relevant disease-promoting and disease-preventive effects of microbial communities on implanted material. The patient group could be applied as a model system for studying the interplay between bacteria on prosthetic implants and the development of inflammatory processes such as capsular contracture. If successful, this project could provide fundamental advances to the use of prosthetic implants that can be extended to other medical fields.
Costerton Biofilm Centre, SUND
University of Copenhagen
Professor Thomas Bjarnsholt
Research unit at the Department of plastic surgery
Rigshospitalet
Professor Emeritus Krzysztof Drzewiecki
 Professor Søren Johannes Sørensen
Professor Søren Johannes Sørensen
I am currently team leader for a group of 12 scientists, 11 phd’s, three technicians and ten master students applying molecular techniques in microbial ecological studies at the Section of Microbiology. My research focuses on social interactions in microbial populations. My group’s studies evaluate the extent of genetic flow within natural communities and their response to environmental perturbations.
The section houses state of the art culture independent experimental infrastructure that enables us to examine how microbes interact at a scale that is relevant for such small organisms and to examine and identify the roles of the individual species that contribute to these interactions.
We investigate how microbes work together in in their natural state such as biofilms and the effect this has on microbial communities in in vivo systems such as human or animal gut, soil, or phase change communities. These are related to our in vitro model systems such as our bioflux biofilm system where we can evaluate our findings and examine phenomenon such as horizontal gene transfer or emergent community properties and evolutionary strategies. To do so we use techniques such as high throughput sequencing, flow cytometry and ultra-high resolution bioimaging or confocal microscopy and microbial reporter systems.
The pioneering work of the section has had been recognized for its impact in diverse areas in which microbial interactions are critical. These include, Soil biodiversity and bioremediation, the role of horizontal gene transfer, human disease progression, the effects of species diversity in plant animal and environmental microbiomes, how microbes interact in evolutionary strategies and the significance of cooperation verses antagonism in microbial systems.
Working in these areas necessitates close collaboration with international scientists from around the world that come to the section and to whom members of the section visit in scientific exchange programs. They span all corners of the globe including Dr. Duncan Veal, Macquarie University, Sydney Australia, Prof A. Spormann, Stanford University, Prof. S. Kjelleberg, University of NSW; Prof. M. Schloter, Helmholtz Zentrum München; and Prof. Md Hans Bisgaard COPSAC back here in Denmark.
These collaborations have helped more than 20 postdocs and 30 past Ph.D. fellows follow their career path both here and around the world to obtain successful positions in both academia and research.
Section of Microbiology

Funded by:
Grant in Clinical and Translational Medicine from the Novo Nordisk Foundation
Project title: The role of low-virulence bacteria located on prosthetic breast implants in the development of capsular contracture
Project period: 01.01.2019 - 31.12.2021
Contact
 Professor
Professor
Søren Johannes Sørensen
Universitetsparken 15
building 1, 1st floor
DK-2100 Copenhagen
Email: sjs@bio.ku.dk

