Neuroendocrinology and Physiology Lab
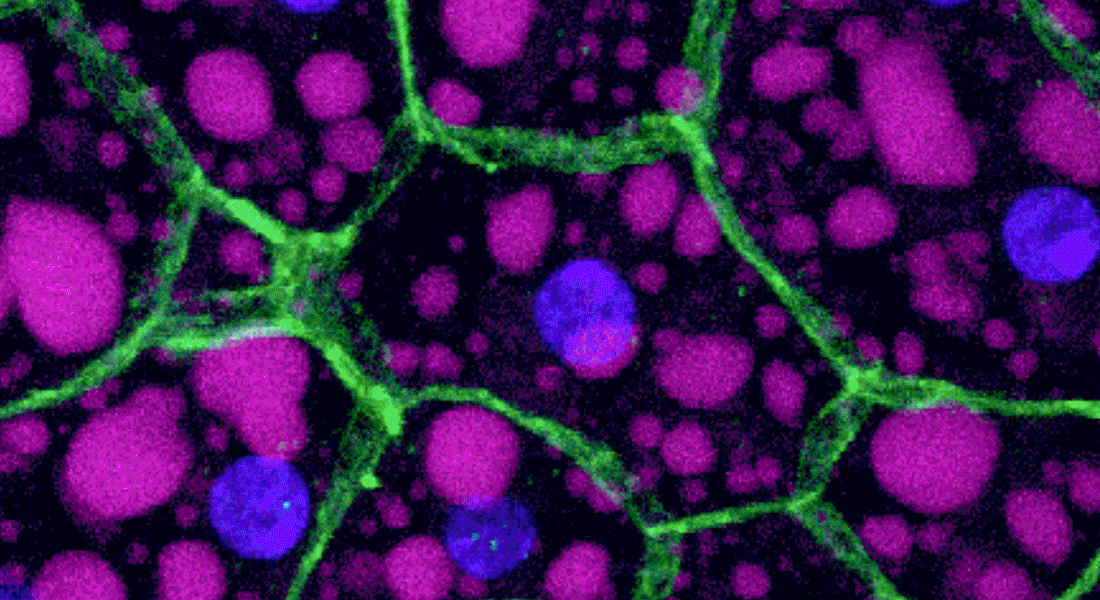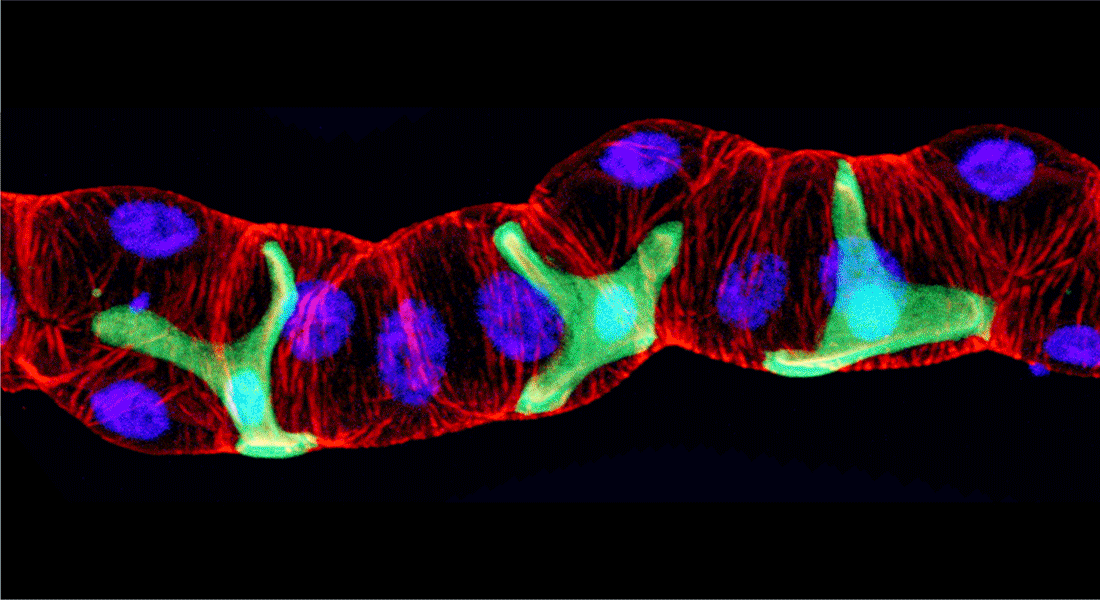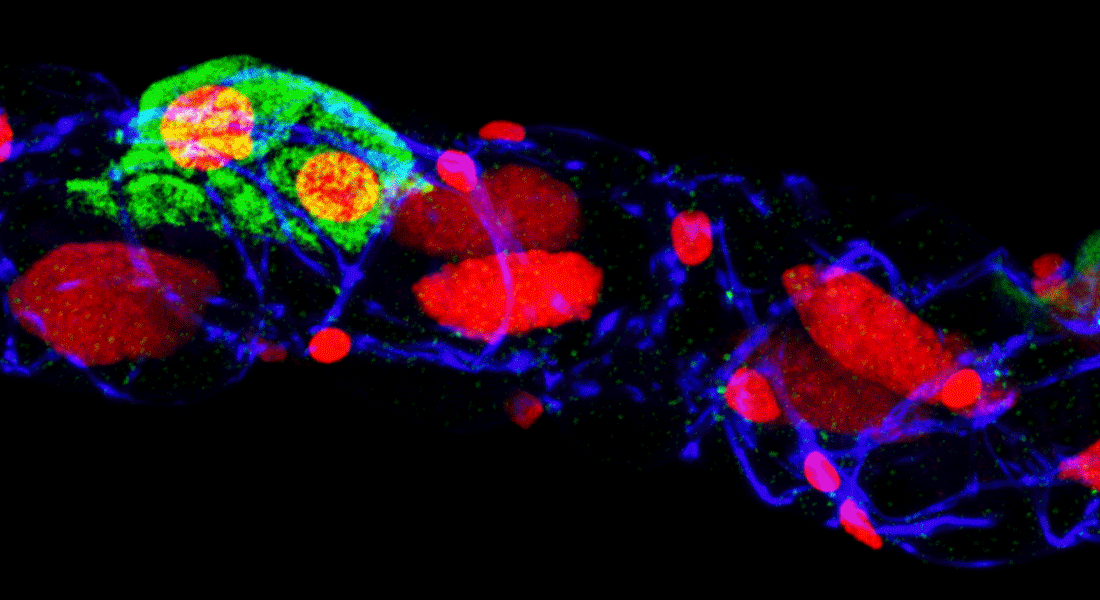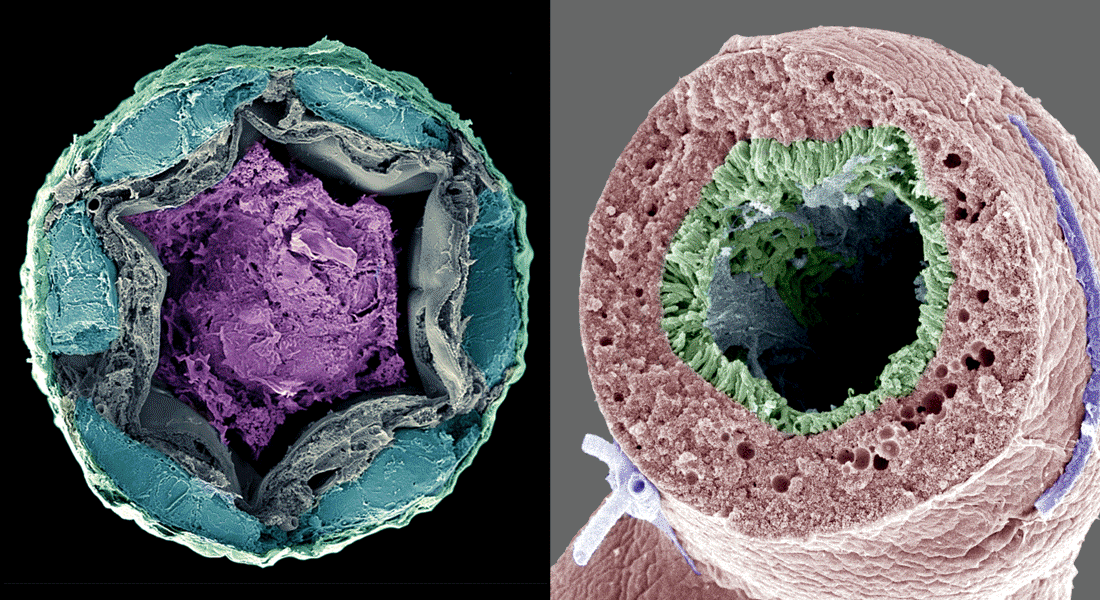
Our overall research focus is to understand the mechanisms by which long range signaling molecules, e.g. neuropeptides and peptide hormones, act as systemic mediators of intercellular communication to direct critical actions in development, metabolism, reproduction and physiology.
We are interested in the endocrine control of adult organs: what are the molecular, cellular and network mechanisms underpinning the functions of specialized organs and how are these regulated by extrinsic signals in response to internal or external challenges. We typically use Drosophila melanogaster or Tribolium castaneum to explore these questions, as they allow us to explore organ communication from an integrative perspective: how different organs (brain, gut, fat body and kidneys) sense and integrate internal and external signals and relay this information to other organs in a manner that ensures a coordinated response by the organism. Our work is highly cross-disciplinary and typically involve a combination of genome editing and/or genetically encoded tools, cutting-edge ‘omics’ technologies, biochemical methods and classic physiological approaches. Beyond gaining insights into the basic underlying biology, our research may important for understanding human metabolic or kidney disorders and for developing novel insect pest control agents.
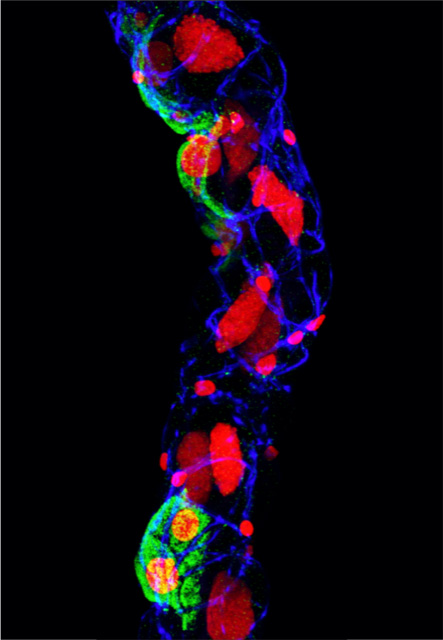
Neuroendocrine Targets for Novel Insect Pest Control
Insects are among the most successful organisms on earth, exploiting and inhabiting the widest possible range of habitats. They also pose the biggest threat to global food security, destroying 15-25% of the total crop yields annually. In this project we seek to explore fundamental question in insect physiology and endocrinology to unmask novel targets for insect biocontrol. Using the red flour beetle, Tribolium castaneum, we are exploring mechanisms of ion and water homeostasis (an attractive target for intervention) to identify and characterize neuroendocrine systems involved in regulating salt and water balance. Recent, our work has uncovered a key neuropeptide system that controls beetle ion and water homeostasis by fundamentally different mechanism than other insects (Koyama et al. 2021 PNAS). This study may help guide the development of novel peptide mimetics that can fatally disrupt this critical life processes.
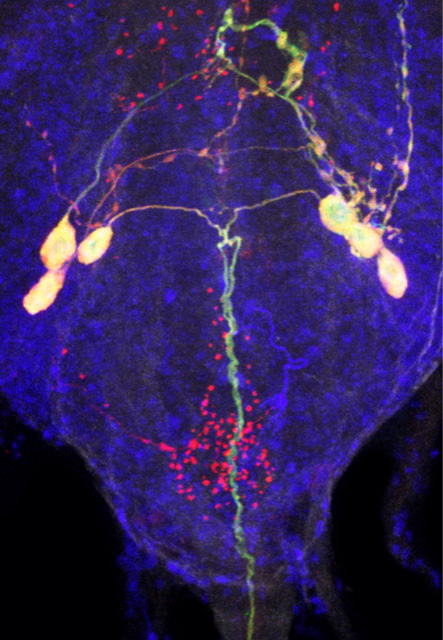
Novel Mechanisms of Osmosensation and Systemic Osmoregulation
Animals must continuously adapt to osmotic challenges to maintain homeostasis and to survive. This requires the coordinated actions of organs with specialized functions, which in turn are modulated by systemic signals communicated by other organs to ensure an appropriate physiological response by the organisms. Molecular osmoreceptors lie at the heart of the central mechanisms coordinating these processes. Yet, the molecular identity of such osmosensitive molecules, and how they modulate the release of known neuroendocrine factors that control systemic osmoregulation, is unknown. Using Drosophila melanogaster for discovery, we aim to gain insights into the molecular, cellular and network mechanisms that control systemic osmoregulation in higher organisms.

Endocrine regulators of AKH/glucagon mediated energy homeostasis
Maintaining biological functions under fluctuating internal and environmental conditions requires the homeostatic control of circulating energy levels. Analogous to mammalian glucagon, the insect adipokinetic hormone (AKH) act as the key hormone in controlling the mobilization of energy reserves during periods of negative energy balance. However, virtually nothing is known about the mechanisms that regulate AKH production and release. We and other have recently shown that the AKH-producing cells are regulated by other systemic factors (Koyama et al. 2021 Nat Comm) raising the possibility that AKH release is under complex control by other extrinsic factors. Using Drosophila genetics in combination with advanced transcriptomic approaches, we aim to gain an authoritative overview of the hormonal pathways that regulate AKH release, which is key to better understand insect metabolism, and more broadly, mechanistic aspects of glycemic control in mammals.

Cell and molecular architecture of a countercurrent exchange system
The insect cryptonephredial complex (CNC) is one of the most powerful water-extraction systems in nature. The essential ‘design principles’ of the CNC lie in the anatomical arrangement of the renal (Malpighian) tubules relative to the rectal epithelia, and on the transport properties of their constituent cells. In effect, the renal tubules build and maintain an osmotic gradient along the length of the rectum allowing the animal extract all water from their excreta to minimize water loss. In some desert species, this mechanism is so efficient that it allows the animal to extract water directly from moist air! In this project, we use Tribolium castaneum as a model to uncover the cellular and molecular architecture underpinning the water reabsorption mechanisms of the CNC. A detailed understanding of CNC function may help inspire biomimicry engineers to design more efficient countercurrent exchange systems, which are broadly used in industry.
CBC Radio (Canada) How beetles absorb water
Beetles can go their whole lives without drinking water through their mouth. Listen to the episode here.
DR.dk Skånsom bekæmpelse af biller
Biller mæsker sig årligt igennem op til en fjerdedel af verdens fødevareforsyning, men nu kan ny viden hjælpe til billebekæmpelse. Læs artikel her.
DRP1 Hjernekassen om skadedyr
Hvilke dyr kalder vi for skadedyr? Hvordan gør de skade? Hvordan bekæmper vi dem i dag? Hvordan bekæmper vi dem i fremtiden? Vi har eksperter i studiet. Hør episode her.
Bananfluers tis gør forskere klogere på menneskets tarme
Hvorfor nogle mennesker rammes af tarmsygdommen Cøliaki, som er forbundet til glutenallergi, er endnu uvist. Men bananfluers urin kan netop have bragt forskerne tættere på at forstå, hvorfor sygdommen opstår. Læs artikel her.
LIST OF PUBLICATIONS IN THE LAST 5 YEARS IN PEER REVIEWED INTERNATIONAL JOURNALS
Co-first authorships are indicated by a double dagger (‡), corresponding authorships are indicated by an asterisk (*), and members from the Halberg lab are listed in italics. Papers listed as “in press” or “accepted” can be sent upon request if not already published by time the application is reviewed. A full publication list is available via Google Scholar.
[35] Leader, D. P., Naseem, M. T., Halberg K. V.* BeetleAtlas: An ontogenetic and tissue-specific transcriptomic atlas for the red flour beetle Tribolium castaneum. Journal of Molecular Biology, Computational Resources, doi.org/10.1016/j.jmb.2024.168520 (2024) | IF 6.151
[34] Halberg K. V. *, Denholm, B. Mechanisms of systemic water balance in insects. Annual Reviews in Entomology. Volume Volume 69: 415-438 (2024) | IF 23.8 | Invited review
[33] Dornan, A. J. ‡, Halberg K. V.‡, L.-K. Beuter, Davies, S.-A., Dow, J. A. T. Compromised junctional integrity phenocopies age-dependent renal dysfunction in Drosophila Snakeskin mutants. Journal of Cell Science jsc.261118 | IF 5.235 | Altmetric = 5
[32] Beaven, R., Halberg K. V., Denholm, B. The insect cryptonephridial system. Current Biology. Volume 33, R743-R759 (2023) | IF 10.900 |
[31] Halberg K. V. *, Rana, D. W., Koyama, T. Managing fuels and fluids: Network integration of osmoregulatory and metabolic hormonal circuits in the polymodal control of homeostasis in insects. BioEssays. 20(7):1-8. (2023) DOI:10.1002/bies.202300011| IF 4.345 | Invited review
[30] Naseem M.T., Beaven R., Koyama T., Naz, S., Su, M., Leader, D., Klærke D., Callø K., Denholm, B., Halberg, K. V.*. NHA1 is a cation/proton antiporter essential for the water-conserving functions of the rectal complex in Tribolium castaneum. PNAS 120:13 1-11 (2023) | IF 11.1 | Altmetric = 278
[29] Malita A.‡, Kubrak O.‡, Koyama T., Ahrentløv N., Texada M. J., Nagy S., Halberg K. V., Rewitz K. A gutderived hormone suppresses sugar appetite and regulates food choice in Drosophila. Nature Metabolism, 4:1532-1550 (2022) | IF 20.8 | Altmetric = 24
[28] Kubrak O.‡, Koyama T.‡, Ahrentløv N., Jensen L., Malita A., Naseem M. T., Lassen M., Nagy S., Texada M. J., Halberg K. V., Rewitz K. The gut hormone Allatostatin C regulates food intake and metabolic homeostasis under nutrient stress. Nature Communications, 13:692 (2022) | IF 16.6 | Altmetric = 24
[27] Koyama T. ‡, Terhzaz S.‡, Naseem M. T., Nagy S., Rewitz K., Dow J. A. T., Davies S.-A., Halberg, K. V.* A nutrient-responsive hormonal circuit mediates an inter-tissue program regulating metabolic homeostasis in in adult Drosophila. Nature Communications, 12:5178 (2021) | IF 16.6 | Altmetric = 24
[26] Koyama T., Naseem M. T., Kolosov D., Vo C. T., Mahon D., Jakobsen A. S. S., Jensen R. L., Denholm B., O’Donnell M., Halberg K. V.* A Unique Renal Architecture in Tribolium castaneum Informs the Evolutionary Origins of Systemic Osmoregulation in Beetles. PNAS, 118:14 1-12 (2021) | IF 11.1 | Altmetric = 99
[25] Koyama, T., Texada, M., Halberg, K., Rewitz, K. Metabolism and growth adaptations to environmental conditions in Drosophila. CMLS, 77:4523–4551 (2020) | IF 9.261 | Altmetric = 6
[24] Christensen, F. C., Koyama, T., Nagy, S., Danielsen, E. T., Texada, M., Halberg, K. A., Rewitz, K. Ecdysonedependent feedback regulation of the prothoracicotropic hormone controls the timing of developmental maturation. Development, 147(14):dev188110 (2020) | IF 6.868 | Altmetric = 10
[23] Maurer, G. W., Malita, A., Nagy, S., Koyama, T., Werge, T., Halberg, K. A., Texada, M. J., Rewitz, K. Analysis of genes within the schizophrenia-linked 22q11.2 deletion identifies interaction of night owl/LZTR1 and NF1 in GABAergic sleep control. PLoSGenetics, 16(4): e1008727 (2020) | IF 5.917 | Altmetric = 17
[22] Persson, D.‡, Halberg, K. A.‡, Neves, R. C., Jørgensen, A., Kristensen, R. M., Møbjerg, N. Comparative myanatomy of Tardigrada: new insights from the heterotardigrades Actinarctus doryphorus and Echiniscoides sigismundi. BMC Evolutionary Biology, 19:206 (2019) | IF 3.045 | Altmetric = 7
[21] Texada, M., Joergensen, A. F., Christensen, C. F. Koyama, T., Malita, A., Smith, D., Marple, D. F. M., Danielsen, T., Petersen, S. K. Hansen, J., Halberg, K.A., Rewitz, K. A fat-tissue sensor couples growth to oxygen availability by remotely controlling insulin secretion. Nature Communications, 10:1955 (2019) | IF 16.6 | Altmetric = 16
[20] Texada, M., Malita, A., Christensen, C. F., Dall, K. B. Faergeman, N. J. Nagy, S., Halberg, K. A., Rewitz, K. Autophagy-mediated cholesterol trafficking controls steroid production. Developmental Cell, 48: 1-13 (2019) | IF 12.270.
LIST OF BOOK CHAPTERS IN THE LAST 5 YEARS IN PEER REVIEWED INTERNATIONAL JOURNALS
[1] Halberg K. V.*, Koyama, T., Denholm, B. Chapter 9. The Malpighian tubule. In: Bernard Moussain (Ed.), Insect Anatomy (Elsevier), pp 9:1-48 (2023).
[2] Dow, J. A. T., Halberg K. A., Terhzaz S. & Davies S. A. Drosophila as a model for neuroendocrine control of ionic homeostasis. In: M. Ludwig & G. Levkowitz (Ed.), Model Animals in Neuroendocrinology: From worm to mouse to man, pp 81-100 (2018).
- Dr. Adam Dobson, Institute of Molecular Cell and Systems Biology, University of Glasgow, UK
- Senior Lecturer Barry Denholm, Centre for Discovery Brain Sciences, University of Edinburgh, UK
- Professor Julian Dow, Institute of Molecular Cell and Systems Biology, University of Glasgow, UK
- Professor Michael O’Donnell, Department of Biology, McMaster University, Canada
- Professor Emeritus David Leader, Institute of Molecular Cell and Systems Biology, University of Glasgow, UK
- Associate Professor, Kirstine Callø, Department of Veterinary and Animal Sciences, University of Copenhagen, DK
- Professor Dan A. Klærke, Department of Veterinary and Animal Sciences, University of Copenhagen, DK
- Professor Kim F. Rewitz, Department of Biology, University of Copenhagen, DK
- Professor Michael F. Romero, Department of Internal Medicine, Mayo Clinic, US
Sensory Biology
https://kurser.ku.dk/course/nbik15019u
Zoophysiology
https://kurser.ku.dk/course/nbia04054u
Comparative Anatomy
https://kurser.ku.dk/course/nbia04004u
Principal subject on molecular biology and immunology
https://kurser.ku.dk/course/NBIK18003U
Basic cell biology
https://kurser.ku.dk/course/nbia04036u
2024 50.000 DKK
Winner of the Food and BioCluster Pitch prize, Århus, DK
2024 1.494.678DKK
(co-PI) InnoExplorer | Innovationsfonden
2024 394.553 DKK
(co-PI) Proof-of-Concept grant (PoC max), UCPH
2023 1.500.000 DKK
(co-PI) Carlsberg Foundation (research infrastructure)
2023 4.995.397 DKK
(PI) Carlsberg Foundation | Carlsberg Accelerate
2023 4.998.584 DKK
(PI) Novo Nordisk Foundation (NNF) | Synergy Program
2023 30.000 DKK
(co-PI) UCPH intramural funding
2023 782.000 DKK
(co-PI) Spin-outs Denmark, Villum Foundation (DK)
2023 2.115.109 DKK
(co-PI) Project grant (BBSRC)
2023 244.992 DKK
(co-PI) GLA(UK)-CPH(DK) strategic collaboration grant (BBSRC)
2022 29.983 DKK
(PI) Ragna Rask-Nielsens Grundforskningsfond
2020 6.191.985 DKK
(PI) Sapere Aude | DFF Research Leader
2020 1.412.955 DKK
(co-PI) Carlsberg Foundation (research infrastructure)
2019 1.824.553 DKK
(co-PI) The Leverhulme trust
2017 200.000 DKK
(co-PI) Carlsberg Foundation (research infrastructure)
2017 8.490.128 DKK
(PI) Villum Foundation| Villum Young Investigator
2015 1.144.500 DKK
(PI) Carlsberg Foundation
2015 68.761 DKK
(co-PI) The Carnegie Trust
2015 942.057 DKK
(PI) Villum Foundation
2013 2.290.108 DKK
(PI) DFF | FNU
Members
| Name | Title | Phone | |
|---|---|---|---|
| Kenneth Veland Halberg | Associate Professor | +4535331155 | |
| Muhammad Tayyib Naseem | Assistant Professor | ||
| Takashi Koyama | Postdoc | +4535334302 |
Contact
The Neuroendocrinology and Physiology Lab
Section for Cell and Neurobiology
Universitetsparken 15
DK-2100 Copenhagen Ø, Denmark
Contact:
Associate Professor Kenneth Halberg
Email: kahalberg@bio.ku.dk
Phone: +45 35 33 11 55
X (Twitter), LinkedIn
| Usama Saeed | PhD Student | |||
