Organ Homeostasis & Physiology Lab

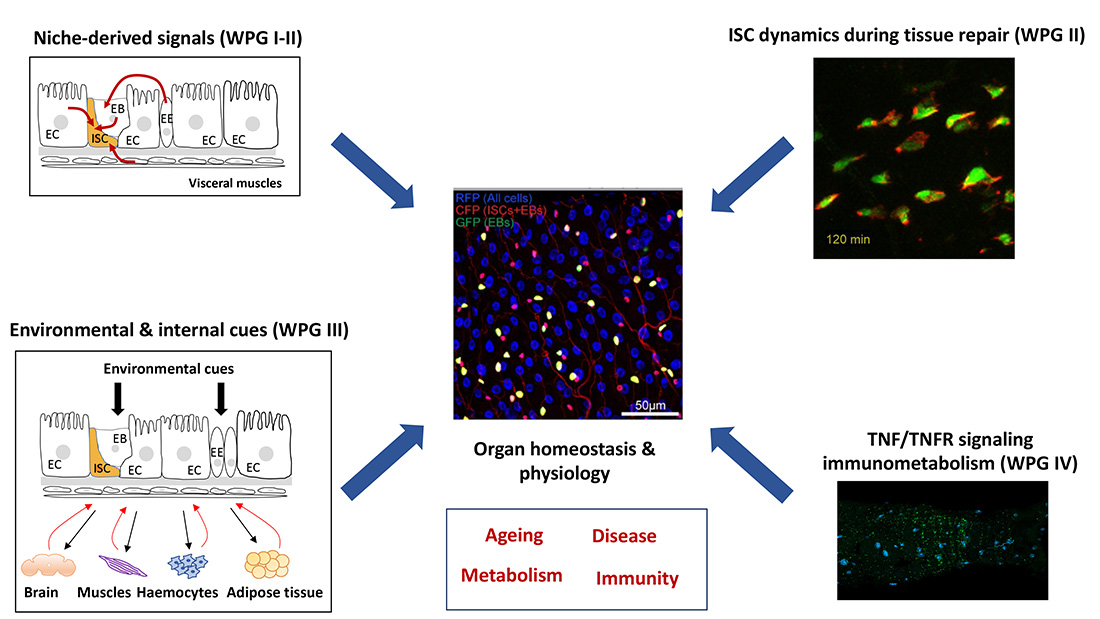
Multicellular organisms have evolved organs and tissues with highly specialized tasks. The function of each organ is modified by local clues and systemic signals derived from other organs to ensure a coordinated response accommodating the physiological needs of the organism.The intestine, which represents one of the largest interfaces with the external environment, plays a key role in relaying environmental inputs to other organs to produce systemic responses. Our team is interested in identifying the intra- and inter-organ couplings contributing to intestinal stem cells biology, gut homeostasis, organism physiology and disease.
Adult tissues with high turnover rates depend on stem cells to provide a continuous source of differentiated cells to maintain tissue homeostasis. To ensure optimal tissue homeostasis and physiology, adult stem cells must coordinate their own maintenance with the generation of differentiated cell types in a temporally and spatially controlled manner. Our team uses the Drosophila gut as a model to study the mechanisms underpinning tissue homeostasis, regeneration, and plasticity. We combine genetic/functional approaches with live imaging of cultured gut explants to decipher the gene regulatory networks that guide intestinal stem cell (ISC) migration, division and fate decisions during regenerative and adaptive growth. We aim to understand how dynamic interactions between intestinal stem cells and their environment faciliate ISC migration during the early phase of tissue regeneration and how homestatic growth patterns are reestablished following repair. Beyond this, we also explore how the gut senses environmental stressors, such as nutrient scarcity, pathogens, or mechanical forces, and and transmits this information to drive appropriate metabolic and behavioral adaptations. Our research focuses on how certain processes are spared, while others are deprioritized, during nutrient scarcity and the trade-offs between immunity and reproduction during pathogenic infections.
Project 1: Mechanisms underpinning adult ISC migration during tissue regeneration
The ability of adult stem cells (SCs) to adopt migratory behavior in response to specific stimuli has attracted significant attention in recent years, given its potential applications in wound repair and regenerative medicine. However, the mechanisms that direct SCs to injured sites and ensure their precise positioning within tissues remain poorly understood. Notably, active migration of somatic SCs is believed to be a critical component of the regenerative response across most adult tissues. To study the process of ISC migration within the context of a living tissue, our team has developed ex vivo culture conditions for wholemount guts, enabling real-time monitoring of ISC migration. Using this approach, we recently showed that the PDGF-VEGF-related ligand, Pvf1, is produced by wound-associated tracheal cells (equivalent of the mammalian vasculature) and act as a chemoattractant to guide ISC migration towards the injured area during tissue regeneration. Live imaging of gut explant provides a platform for exploring the role other signals, including mechanical cues, in controlling ISC migrations during tissue regeneration. Our research focuses on how dynamic interactions between ISCs and their environment facilitate ISC migration during the early stages of tissue regeneration. As pro-migratory signals involved in tissue healing are often subverted by cancer cells for invasion and dissemination, we are also developing intestinal tumor models to study the role of these signals in cancer cell progression.
Project 2: Balancing lateral inhibition and tissue plasticity
Delta/Notch mediated lateral inhibition is a process that prevent neighboring cells from adopting the same fate. It plays a critical role in creating spatial patterns of cell types during development and within adult tissues. Lateral inhibition is established through an amplifying feedback loop, where Delta activates Notch in adjacent receiving cells (Notch ON), which suppresses Delta expression in these cells thereby reducing Notch activity in Delta expressing cells (Notch OFF). Homeostatic growth patterns are maintained through lateral inhibition between ISCs and their progeny, which maintains an appropriate number of SCs and balances cell fate decisions. By contrast, following injury, homeostatic patterns correlating with large differences in Notch activity are temporarily disrupted to increase ISC motility and numbers to promote tissue regeneration. Our previous work demonstrated that epithelial damage induces a shift from the evenly distributed ISC patterns characteristic of homeostasis to the formation of regenerative ISC clusters, driven by the recruitment of ISCs to injured sites. Crucially, after repair, the increase in cellular plasticity and disruption of spatial ISC patterns must be complemented by the reverse process to restore tissue architecture and prevent unrestricted stem cell divisions and tumor formation. Importantly, how Delta/Notch mediated lateral inhibition and cellular plasticity are balanced during these transitions remains poorly understood. By combining functional genetics and real time imaging of gut explants to track ISCs dynamics, our team investigates the mechanisms governing the dynamics and positioning of ISC within the epithelium during regenerative growth and upon return to homeostatic growth patterns.
Project 3: Mechanical forces in adult tissue homeostasis, repair, and disease
Epithelial cells enveloping our organs are constantly exposed to mechanical forces, such as blood shear pressure, lung inflation, or expansion of the bladder or gut. Nevertheless, it remains poorly understood how mechanical forces impact on adult organ homeostasis and physiology. The adult gut, a highly dynamic tissue subjected to shear stress, compressions, and swellings from luminal content, serves as an excellent model for studying how adult stem cells adapt their activity and fate to accommodate mechanical perturbations. While mechanical forces are known to influence gut epithelial dynamics and composition, the mechanisms linking luminal forces to changes in ISC behavior are less clear. To explore how mechanical forces are sensed and converted into tissue-scale adaptive responses, we have developed a range of ex vivo and in vivo experimental approaches that enable us to monitor the effects of mechanical stretch on single-cell behavior and tissue-scale dynamics.
Mechanical competition between tumours and the surrounding tissues is thought to play a critical role in tumour progression and dissemination, and consistent with this, mechanosensitive pathways are deregulated in a wide range of solid and non-solid tumours. We are developing intestinal tumour models to probe the mechanical properties of tumours (e.g. stiffness) and their microenvironment. These models allow us to test the requirement for our mechanosensitive candidate genes in promoting tumour growth and dissemination.
Project 4: Trade-offs
Higher brain functions, such as social cognition, memory, and learning, require significant metabolic energy and neural connectivity. While these abilities provide long-term advantages, such as improved social cooperation and environmental adaptation, they may come at the expense of fundamental survival behaviors (such as eating, fleeing, or mating). In conditions of limited resources, energy normally allocated to higher brain functions is redirected to support essential survival-oriented behaviors, such as foraging, and metabolic adaptation. Our team explores the molecular mechanisms balance this trade-off between higher cognitive functions and pro-survival behaviors during periods of nutrient scarcity.
Investing in immunity is critical for survival, especially in environments rich in pathogens. However, maintaining a robust immune system is energetically costly and often diverts resources away from reproduction. For example, during infection or inflammation, animals may experience reduced fertility or delay reproductive efforts. Our team explores how the presence of pathogens in the gut lumen is sensed and coupled with effects on reproductive output. As stress-induced immune activation often suppresses reproductive functions, such insight could guide interventions to balance immune responses and optimize conditions for conception.
Project 5: The role of TNF/Egr in adapting metabolism and behavior to external stressors or changes in the internal physiological state
Our team has a long-standing interest in understanding the physiological and pathophysiological of tumor necrosis factor (TNF). TNF is released from adipose tissues, endothelial cells, and activated immune cells, serving as a key mediator of the inflammatory responses associated with chronic infections and inflammatory diseases. Beyond its classical immune and pro-inflammatory functions, TNF is increasingly recognized as an important metabolic messenger and driver of metabolic disease associated with chronic inflammatory conditions, such as cancers and type 2 diabetes. While growing evidence suggest that TNF plays an instrumental role in orchestrating the metabolic rewiring associated with pathophysiological conditions, less is known about the physiological role that TNF serve to promote energy homeostasis in healthy individuals. The wide-spread use of anti-TNF therapies to treat chronic inflammatory diseases, such as inflammatory bowel disease (IBD), highlights the urgency to better understand the physiological/beneficial functions of TNF and how these might be affected by anti-TNF drug regimes. The functional redundancy and the complexity of the mammalian TNF ligand-receptor network, which is composed of 19 ligands and 29 related receptors, constitute an obstacle to uncover the physiological functions of TNF. In contrast, the Drosophila TNF receptor system comprising a single ligand, Eiger (Egr), and two receptors, Grindelwald (Grnd) and Wengen (Wgn), provides a simpler model for investigating TNF’s role in energy homeostasis in healthy individuals. Using this model, we have previously shown that the TNF receptor, Wgn, is critical for linking immune responses with lipid metabolism to promote tissue homeostasis in the adult gut. Building on this work, we are now exploring the role of TNF in regulating systemic energy homeostasis.
2025
The innate immune system: A double-edged sword
.
2024
Pvf1-PvR-mediated crosstalk between the trachea and the gut guides intestinal stem cell migration to promote gut regeneration.
.
Wengen's hidden powers: ROS triggers a TNFR-dependent tissue regenerative pathway in Drosophila.
Ditte S. Andersen & Julien Colombani
The EMBO Journal, 43(17), 3550-3552.
Drosophila activins adapt gut size to food intake and promote regenerative growth.
Christian Fokdal Christensen, Quentin Laurichesse, Rihab Loudhaief, Julien Colombani, Ditte S. Andersen.
Nat Commun. 2024 Jan 4;15(1):273. doi: 10.1038/s41467-023-44553-9.
2023
The Drosophila Tumor Necrosis Factor Receptor, Wengen, couples energy expenditure with gut immunity.
Rihab Loudhaief, Rouba Jneid, Christian Fokdal Christensen, Duncan Mackay, Ditte S. Andersen, Julien Colombani.
Sci Adv. 2023 Jun 9;9(23):eadd4977. doi: 10.1126/sciadv.add4977.
Drosophila TNF/TNFRs: At the crossroad between metabolism, immunity, and tissue homeostasis.
Colombani, J. & Andersen, D.S.
FEBS Lett. 2023 Oct;597(19):2416-2432.
2022
A Dilp8-dependent time window ensures tissue size adjustment in Drosophila.
Blanco-Obregon, D., El Marzkioui, K., Brutscher, F., Kapoor, V., Valzania, L., Andersen, Ditte Skovaa, Colombani, Julien, Narasimha, S., McCusker, D., Léopold, P. & Boulan, L.
Nat Commun. 2022 Sep 26;13(1):5629. doi: 10.1038/s41467-022-33387-6.
2021
Drosophila TNFRs Grindelwald and Wengen bind Eiger with different affinities and promote distinct cellular functions.
Palmerini, V.; Monzani, S.; Laurichesse, Q.; Loudhaief, R.; Mari, S.; Cecatiello, V.; Olieric, V.; Pasqualato, S.; Colombani, J.;
Andersen DS. & Mapelli, M.
Nat Commun. 12, 2070 (2021). https://doi.org/10.1038/s41467-021-22080-9.
2020
The Drosophila gut: a gatekeeper and coordinator of organism fitness and physiology.
Colombani, J. & Andersen, D.S.
Wiley Interdiscip Rev Dev Biol., Vol. 49, 2020 Mar 16:e378. doi:10.1002/wdev.378.
2015
The Drosophila TNF receptor Grindelwald couples loss of cell polarity and neoplastic growth
Andersen, D.S.; Colombani, J.; Palmerini, V.; Chakrabandhu, K.; Boone, E.; Röthlisberger, M.; Togweiler, J.; Basler, K.; Mapelli, M.; Hueber, A.O.; Léopold, P.
Nature, Vol. 522, No. 7557, 25.06.2015, p. 482-6.
2012
Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing
Colombani, J.; Andersen, D.S.; Léopold, P.
Science, Vol. 336, No. 6081, 04.05.2012, p. 582-5.
Carlsberg Foundation – Carlsberg Accelerate 2025-2029
Independent Research Fund Denmark – Natural Sciences 2025-2028
Independent Research Fund Denmark – Medical Sciences 2025-2027
Horizon Europe MSCA European Postdoctoral Fellowship - Alphy John 2024-2026
Novo Nordisk Foundation Project Grants in Bioscience and Basic Biomedicine 2021-2023
Novo Nordisk Foundation Young Investigator Award 2020-2026
ERC Starting Grant 2019-2024
Adult tissues with high turnover rates, such the intestine, depend on stem cells (SCs) to provide a continuous source of differentiated cells to maintain tissue homeostasis. To ensure optimal tissue homeostasis and physiology, adult stem cells must coordinate their own maintenance with the generation of differentiated cell types in a temporally and spatially controlled manner. Due to its remarkable self-renewing capacity, the fly gut has recently become a prime paradigm for studying stem-cell function during adult tissue homeostasis. This capacity for self-renewal relays on the proliferative activity of the intestinal stem cells (ISC), which is tightly coupled with cell loss to maintain intestinal homeostasis. ISC proliferation is controlled by multiple local and systemic signals released from the ISC niche (enterocytes (ECs), enteroendocrine cells (EECs), enteroblasts (EBs), and visceral muscles (VMs)) and non-gastrointestinal organs. Deregulation of ISC proliferation affects gut integrity, which in turn can trigger several chronic inflammatory diseases including colorectal cancer. Identifying niche-derived and systemic signals controlling gut epithelial turnover therefore represents an important step towards treating gut inflammatory diseases. The project takes advantage of the genetic amenability of the fruit fly to identify ISC niche-derived and systemic signals that control ISC activity and gut homeostasis.
While TNF signaling has mainly been studied in relation to its pathological role in driving inflammation-related metabolic disease, it is not clear whether TNF/TNF receptor (TNFR) signaling controls energy homeostasis in healthy individuals. We found that the highly conserved Drosophila TNFR, Wengen (Wgn), is required in the enterocytes (ECs) of the adult gut to restrict lipid catabolism, suppress immune activity, and maintain tissue homeostasis. Our findings suggest that Wgn/TNFR functions as an intersection between metabolism and immunity allowing pathogen-induced metabolic reprogramming to fuel the energetically costly task of combatting an infection (Loudhaief et al 2023).
To identify ISC niche-derived signals that are required for gut homeostasis and/or infection-induced regeneration, we selected all secreted peptides (app 800) and receptors (app 600) expressed in the adult gut, performed adult-specific knockdown of these in different gut resident cell populations using RNAis, and screened for increased sensitivity to oral infection with the mildly pathogenic bacteria Ecc15. Among the candidate genes identified in the primary screen, we conducted a secondary screen on the top 46 (secreted peptides) and 69 (receptor) hits, which were selected based on reproducibility and conservation in higher organisms. For this screen, we evaluated the effects of knocking down candidate genes on tissue turnover in homeostatic conditions (WPG I + III) and the proliferative response triggered by intestinal infection (II-III). Among our top candidate hits, we identified the two Drosophila activin ligands, Activin- (Act) and Dawdle (Daw) as key regulators of adult gut homeostasis. In short, we found that Act and Daw control distinct steps of intestinal stem cell (ISC)-to-enterocyte (EC) maturation and couple different environmental cues with the appropriate adaptive responses. While Act is highly upregulated in EBs in response to infection and required for the accelerated tissue turnover associated with regenerative growth, Daw responds to nutritional cues and plays an essential role in the adaptation of organ size to nutrient intake. This work was presented at several internal conferences (as selected talk) and published in Nature Communication in January 2024 (Christensen et al 2024).
Our screens also identified the highly conserved PDGF-VEGF-related ligand, Pvf1, and its receptor, PDGF-VEGF-related receptor, Pvr, as critical regulators ISC migration during gut regeneration. (WPGI-III). This work was presented as talks at multiple international conferences and will be submitted for publication within the next few months.
Finally, we could show that the highly conserved Drosophila TNFR, Wengen (Wgn), is required in the enterocytes (ECs) of the adult gut to restrict lipid catabolism, suppress immune activity, and maintain tissue homeostasis. Wgn limits autophagy-dependent lipolysis by restricting cytoplasmic levels of the TNFR effector, TNFR-associated factor 3 (dTRAF3), while it suppresses immune processes through inhibition of the dTAK1/TAK1-Relish/NF-κB pathway in a dTRAF2-dependent manner. This suggests that Wgn/TNFR functions as an intersection between metabolism and immunity allowing pathogen-induced metabolic reprogramming to fuel the energetically costly task of combatting an infection. Our work highlights the important protective and metabolic functions TNFRs might serve in the gut of healthy individuals and raises the question as to how the widespread use of anti-TNF therapies in the treatment of chronic inflammatory diseases, such as inflammatory bowels disease might affect these. This work was presented at several international conferences (as selected talk) and published in Science Advances last year (Loudhaief et al 2023).
The capacity of the intestine to adapt its size according to nutrient availability is highly conserved and particularly evident in animals with intermittent feeding patterns. Nevertheless, the mechanism underpinning nutrient-dependent adult gut resizing is not known. One of the most striking observations we made, was that this resizing is mainly controlled at the EB-to-EC differentiation step. Hence, while the pool of intestinal stem cells and mature cells (ECs and EECs) decrease upon nutrient deprivation, we observed an increase in the pool of EB progenitors, which is normalized upon refeeding. Hence, we speculate that the pool of stalled EBs provide a rapid source of ECs allowing quick expansion of the gut upon refeeding (Christensen et al 2023). This study provided the first example of a role of activin signaling in controlling gut homeostasis and points to a critical function of activins in controlling adult tissue homeostasis.
In our study on the role of Wgn/TNFR in controlling gut metabolism and immunity (WPG IV), we uncovered an unexpected TNF-independent role of Wgn in regulating the degradation of Drosophila TNFR-associated factor 3 (dTRAF3) (Loudhaief et al 2023). Intriguingly, another group subsequently published a role of Wgn in controlling the stability of receptor tyrosine kinases in the embryonic tracheal network, suggesting that Wgn/TNFR might have a more general role in controlling protein degradation. Our work opens the exciting possibility that other TNFRs might regulate protein localization and/or degradation and thereby regulate a broad spectrum of physiological processes independent of their canonical ligands.
Funded by:
 |
 |
Members
| Name | Title | Phone | |
|---|---|---|---|
| Alphy John | Postdoc | +4535334576 | |
| Cecilie August Jensen | PhD Fellow | +4535332532 | |
| Dang Dong Nhi Nguyen | PhD Fellow | +4535326575 | |
| Ditte Skovaa Andersen | Associate Professor | +4535326878 | |
| Elizabeth Catherine Connolly | Postdoc | +4535334586 | |
| Julien Colombani | Associate Professor | +4535320933 | |
| Rouba Jneid | Postdoc | +4535334585 |
Contact
Organ Homeostasis and Physiology Lab
Section for Cell and Neurobiology
Universitetsparken 15
DK-2100 Copenhagen Ø, Denmark
Associate Professor
Ditte S. Andersen
Associate Professor
Julien Colombani
Funded by