Welcome to the Heiðarsson group!
Our research focuses on transcription factors, dynamic protein molecules that regulate genomic architecture and transcription of genes. We study how the structure and dynamics of transcription factors allow them to navigate and modulate the genome, and how they enable cell fate decisions to be made. We are particularly invested in deciphering the activity of pioneer transcription factors involved in cell reprogramming and applying rational design approaches to harness these factors for efficient cell fate control.
We use an integrative approach by combining single-molecule and ensemble techniques, biochemistry, and computer simulations through collaborations, to obtain a microscopic molecular view of transcription factor function.
At BMS, our lab is part of the Structural Biology and NMR Laboratory and the Protein Biology Group. We are also affiliated with the Science Institute and the Biomedical Center, both within the University of Iceland.
Contact Pétur if you are interested joining our team – we always welcome applications! You can read more about current research projects below and read more about joining the lab as a student on the You can also visit our lab website at www.heidarsson.hi.is.
Disordered proteins in regulating access to the genetic material
As organisms become increasingly complex, so too must they evolve a more sophisticated molecular alphabet. The recent discovery of proteins that can adopt multiple structural states is one way of addressing this complexity and it has dramatically changed our view of the protein structure-function paradigm. Almost two thirds of the human proteome is predicted to consist of such proteins that contain long intrinsically disordered regions (IDRs) and therefore lack a stable, well-defined three-dimensional structure. These so-called intrinsically disordered proteins (IDPs) have sometimes been referred to as belonging to the dark proteome since they are on the edge of what traditional structural biology techniques can capture. IDPs are prevalent in cellular regulation and signalling processes, and are implicated in a vast array of diseases and pathologies. We study a wide spectrum of IDPs in the nucleus and aim to obtain a quantitative description of their dynamic structural ensembles to understand function.
Dynamic structural biology of pioneer transcription factors
Transcription factors are particularly enriched in structural disorder where almost all of the ~1600 human factors contain long IDRs. Transcription factors usually have small and folded domains responsible for binding specific DNA sequences. The much longer IDRs contain the transcriptional activation domains and other regions important for binding other proteins and integrating the transcriptional machinery. Deciphering the sequence grammar of transcription factor IDRs and how it dictates structure, dynamics, and function, is one of the primary goals of our lab.
A large focus in our lab Is on pioneer transcription factors; these can bind and open condensed chromatin, and initiate cell identity changes. We use single-molecule approaches to map the structure and dynamics of the factors to understand their interactions with chromatin, and we monitor their effects on chromatin structure. We then use biochemistry and synthetic biology to modulate their function. Finally, we observe their functions and reprogramming abilities using methods from in vitro to living cells.
Chromatin constructs for single-molecule studies
To study transcriptional regulation, we design and develop biochemical approaches for producing chromatin constructs, especially for single-molecule studies. We are currently developing methods to site-specifically label nucleosome arrays with defined transcription factor binding sites, to monitor chromatin structure during transcription factor binding. Most chromatin research has relied on using very stable DNA sequences that are not normally found in organisms. We are therefore also devoted to developing approaches to use „native“ DNA sequences for nucleosome formation, to understand transcription under increasingly native-like conditions.
Pétur directs the project PIONEER, funded through a Starting grant from the European Research Council (ERC) until 2028. The project goal is to map the structural dynamics and kinetics of key pioneer transcription factors, engineer variants for modified functional properties, and to visualize and test their chromatin remodelling and reprogramming abilities, in vitro and in living cells.
The Heiðarsson lab is also part of the NNF Challenge Center REPIN with four other research groups (Birthe Kragelund, Karen Skriver, Rasmus Hartmann-Petersen, and Ben Schuler). The aim of the Center is to rethink protein-protein interactions to encompass the emerging concepts afforded by intrinsically disordered proteins.
Besides these main projects, the group is also involved in research with diverse protein targets, such as classical transcription factors, chaperones, chromatin remodellers, and histone proteins.
Our core technique is single-molecule spectroscopy, usually in combination with Förster resonance energy transfer (FRET). Single-molecule FRET is a sensitive molecular ruler that allows us to measure molecular distance distributions and dynamics on a broad timescale from picoseconds to hours, through multiparameter analysis of photon statistics. The experiments are performed on either freely diffusing molecules, which restricts dynamic processes to the millisecond range or on surface-immobilized molecules which allows conformational or binding kinetics to be measured on a timescale from milliseconds to minutes. We also rely on ensemble methods such as nuclear magnetic resonance (NMR) spectroscopy for atomic–resolution information on protein behaviour. When combined with molecular simulations, these methods can give us a detailed view into biomolecular structure, dynamics, and function. We strive to link molecular behaviour from in vitro to in vivo, by ultimately using fluorescence imaging in live cells as well as genome-wide approaches to study chromatin accessibility.
In our lab at UCPH, we have a Luminosa confocal fluorescence microscope from PicoQuant. This microscope has four picosecond pulsed lasers (485 nm, 530 nm, 595 nm, and 640 nm) and four single-photon avalanche detectors, enabling analysis of fluorescence lifetimes, anisotropy, single-molecule FRET, (ns)FCS, stoichiometry, and more. It is equipped with both piezo and galvo scanners, optimized for confocal fluorescence of surface-immobilized molecules and fluorescence lifetime imaging (FLIM).
We also have a FLEX confocal fluorescence microscope from Exciting Instruments, capable of single-molecule detection, FRET and FCS. This instrument has three continuous-wave lasers (488 nm, 520 nm, 640 nm) enabling alternating laser excitation (ALEX) measurements. At our lab in Reykjavík, we operate a MicroTime 200 confocal fluorescence microscope with two picosecond pulsed lasers (520 nm, 640 nm) and four single-photon avalanche detectors (SPADs). We also have access to the cOpenNMR Center that houses four high-field NMR spectrometers, as well as a suite of instruments for biophysical and biochemical characterization of biomolecules (ITC, DSC, CD, MS, fluorescence, UV-VIS, and more).
We have a wide network of collaborators spanning over three continents, that enable us to bridge diverse fields of science. Some of our closest current collaborations are with Dr. Davide Mercadante, University of Auckland, NZ; Dr. Kenneth Zaret, PENN Institute for Regenerative Medicine, University of Pennsylvania, US; Dr. Vaclav Veverka, Charles University, CZ; Eiríkur Steingrímsson, University of Iceland, IS; Dr. Ciro Cecconi, Dr. Giorgia Brancolini, University of Modena and Reggio Emilia, IT; and the REPIN Consortium that includes Dr. Birthe Kragelund, Dr. Karen Skriver, Dr. Rasmus Hartmann-Petersen, University of Copenhagen, DK, and Dr. Benjamin Schuler, University of Zurich, CH.
Our research is currently funded by grants from the European Research Council (ERC), the Icelandic Centre for Research (Rannís), The University of Copenhagen, University of Iceland, and the Icelandic Cancer Society.
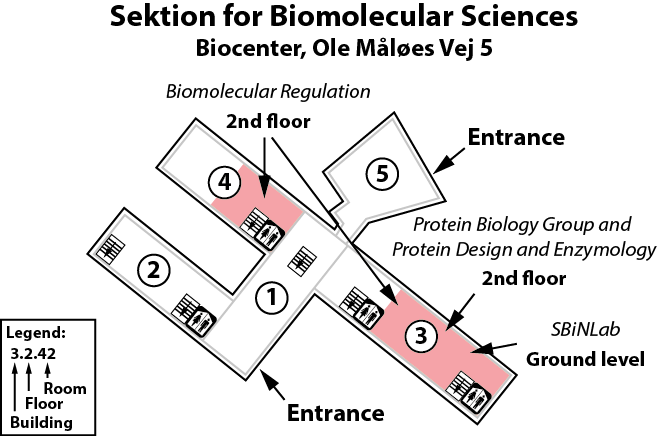
The Heiðarsson lab is located on the 2nd floor, building 3 of the Copenhagen Biocenter (map) close to the centre of Copenhagen. It takes less than 1 hour to travel from Copenhagen's airport to our lab, by train or subway (from the airport to Nørreport station, a 20 minute journey, with trains every 10 minutes) and bus (bus 150S from Nørreport to the stop called "Fredrik Bajers Plads" in the intersection of Tagensvej and Nørre Allé, a 10 minute journey, with buses every 6-8 minutes). From there it is a few minutes walk.
Contact

Associate professor Pétur O. Heiðarsson
Section for Biomolecular Sciences
The Kaj Ulrik Linderstrøm-Lang Centre for Protein Science
Structural Biology and NMR laboratory
Ole Maaløes Vej 5, room 3-2-11
DK-2200 Copenhagen N
Email: poh_at_bio.ku.dk
Phone: +45 3532 3363
KU profile: Link