Garm Lab
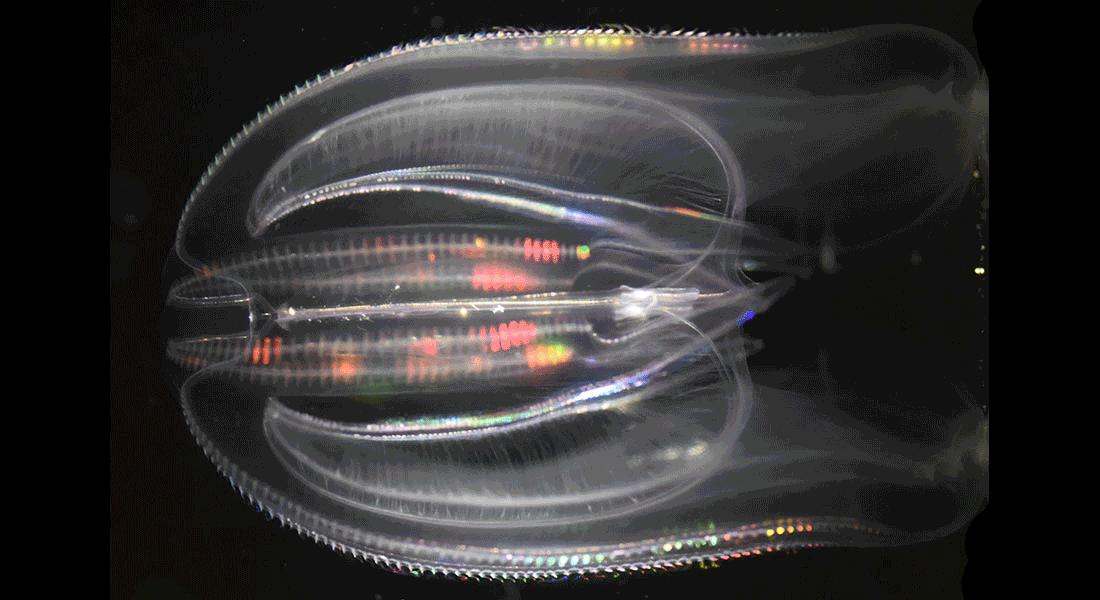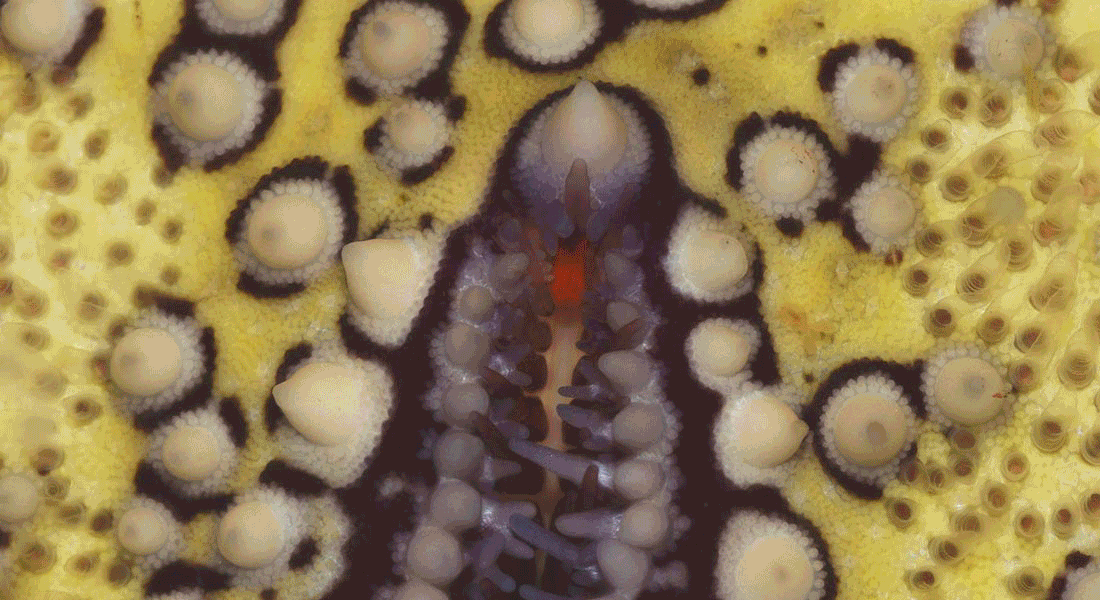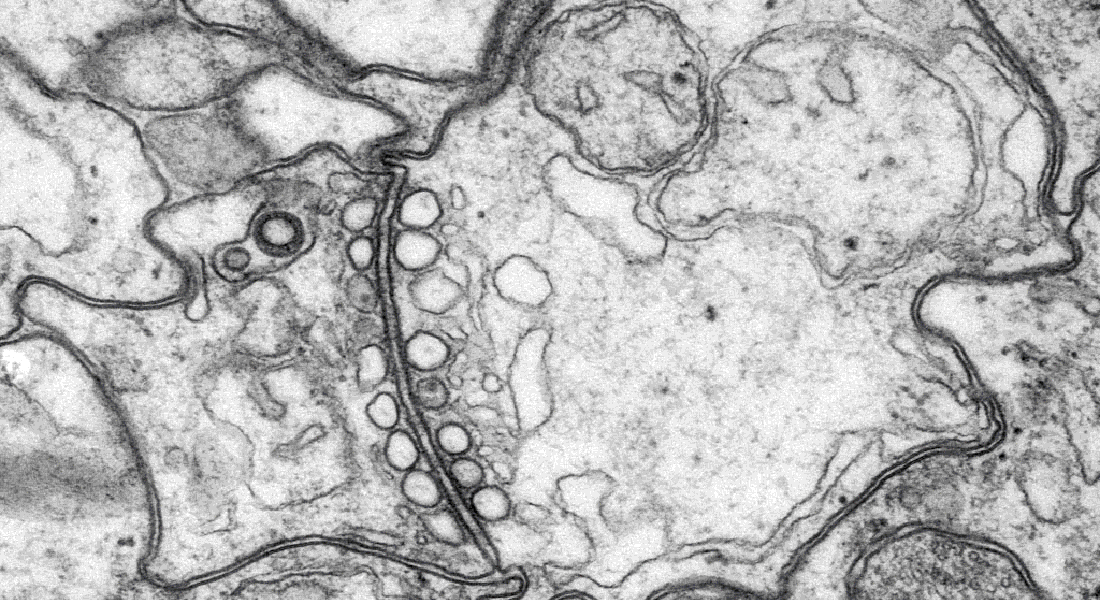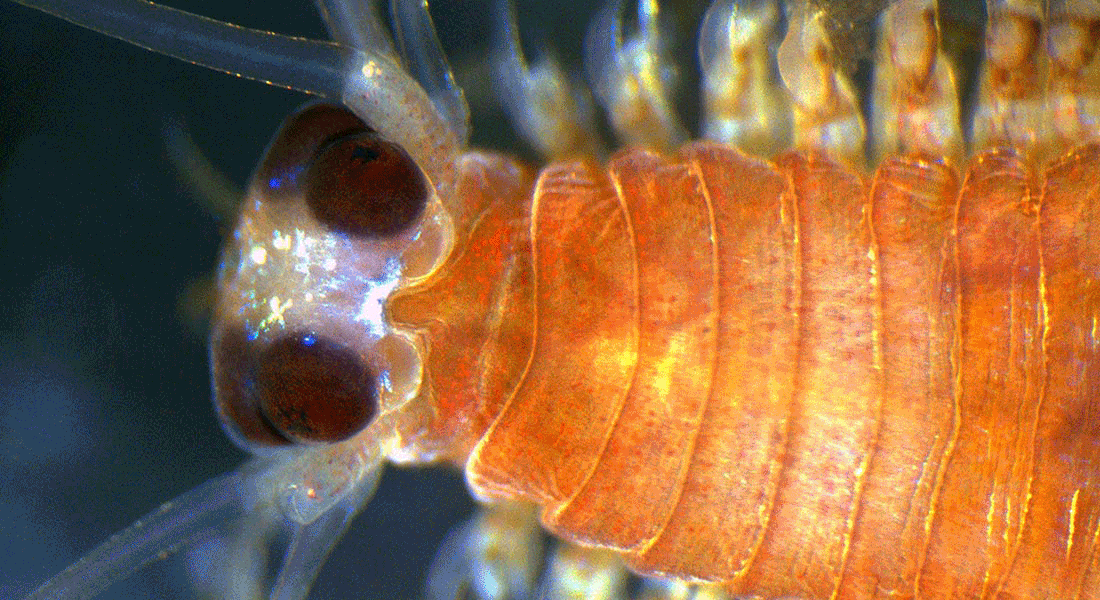
In my group, we study the structure and function of sensory organs and how the information they pick up is processed and turned it into behaviour.
Most of the time, we study the eyes and vision of marine invertebrates such as box jellyfish, starfish, annelids and crustaceans, but we have also worked on mechano-, chemo-, electro- and magnetoreception, respectively.
We use a multiangled approach to study these systems; the key methods we use are electrophysiology (intra and extracellular), microscopy (LM, TEM, SEM, and CLSM), culturing, behaviour (in situ and in vitro) and transcriptomics.
We are particularly interested in how animals with very limited nervous systems seem to be able to process complex information and control a broad range of behaviour. This has led us to work on ‘compound matched filters’, i.e., when a number of narrow band filters work in cooperation to ensure that only the essential information that needs to be processed reaches the CNS. These band filters can be implemented at all levels, from the receptor molecules to the animal’s expressed behaviour.
Another highly prioritized topic in my group is the functional organization of the enigmatic repetitive and dispersed CNS of radially symmetric animals (such as jellyfish and starfish).
Neurobiology and visual ecology of box jellyfish
Within cnidarians (e.g. corals and jellyfish) box jellyfish stand out by displaying an elaborate behavioural repertoire of which most are visually guided.
Utilizing our cultures of the Caribbean species, Tripedalia cystophora, we want to understand precisely what visual information is picked up by their 24 eyes (of which eight are image forming) and how it is processed by their CNS.
We have uncovered several matched filters that come together to perform highly specialized narrow band filtering. Their radially symmetric CNS holds four parallel subunits, called rhopalial nervous systems, RNS, which process the filtered information. Each RNS has a mere 1000 neurons and we study their circuitries using electrophysiology, electron microscopy, genomics, connectomics, transcriptomics and other techniques.

Main collaborators: Todd Oakley (Uni of California) and Jan Bielecki (Christian-Albrechts Uni.)
Neurobiology and visual ecology of starfish
Most examined starfish has a small compound eye at the tip of each of their arms normally having less than 300 ommatidia each. They sit as direct extensions of the radial nerves, which make up the most of the CNS in starfish.
We want to understand the visual capacity of these eyes and have used the coral pest Acanthaster planci (crown-of-thorns starfish) as our model species.
The project also examines how starfish vision changes with different habitat and feeding behaviour and this includes work on several deep-sea species. We also study the structure and function of their radially symmetric nervous system and our working hypothesis is that the radial nerves are parallel brains where most of the visual processing happens.
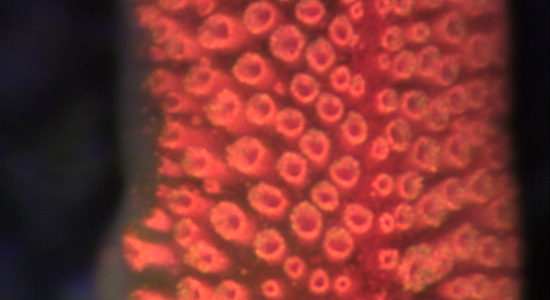
Main collaborators: Dan-E Nilsson (Lund Uni.) and Chris Mah (Smidtsonian Institutute of Marine Science)
Vision in marine annelids
Marine annelids (polycheats) display a broad range of eye types from simple ocelli with only a few cells to large image forming camera-type eyes and compound eyes. The structure, including ultrastructure, is well known but little is known about the receptor physiology and functional significance of the eyes.
We study these aspects of annelid vision using scale worms, fan worms, alciodopids and Platyneries dumerilii. Of particular interest is why several marine annelids have two pairs of prostomial eyes, visual adaptations to detect bioluminescence, visual ecology of alciodopids, and changes in eye physiology during sexual metamorphosis in Platyneries.
Main collaborators: Mike Bok (Lund Uni.), John Kirwan (Uni. Naples) and Katrine Worsaae (Uni. Of Copenhagen)
Dynamics of naturally occurring bioluminescence
Bioluminescence is the ability of living organisms to produce light through biochemical reactions. It is ommi-present in the ocean but is especially important in the open waters where studies have shown that approx. 75% of the animals found here are bioluminescent.
In almost all studies of bioluminescence the light production is artificially evoked, though, typically through mechanical disturbance and little is known about the naturally occurring light events. Further, a whole range of different functions of bioluminescence has been suggested incl. communication, camouflage, lures, and decoys, but in many cases experimental proof is lacking.
We study the naturally occurring bioluminescence in the deep-sea benthos with a focus on brisingid starfish, where some species are bioluminescent and have large image forming eyes, some are bioluminescent but eyeless. Comparing these groups will not only help understand the functional significance of their bioluminescence but also potential visual adaptations to detect the light. We also study the invasive ctenophore, Mnemiopsis leidyi, which is brightly bioluminescent to understand the connection between detailed dynamics of the light emission and function.
Main collaborators: Henrik Glenner (Uni of Bergen), Sönke Johnsen (Duke Uni.), Cornelia Jaspers (DTUaqua), and Jakob Winther (Uni. of Copenhagen)
Light reception and behaviour in dinoflagellates
How advanced does a single cell get? This question is probably best answered through studies of dinoflagellates. We study the dinoflagellate family Warnowiaceae to try and understand these highly advanced single celled organisms.
They are either heterotrophic or mixotrophic feeding on other microorganisms and there are indications that the prey items are detected visually. They have a cell organelle (the ocelloid) resembling an eye and other organelles (nematocysts) resembling harpoons.
We want to understand the functional significance of these organelles during prey capture and how potential image information is processed and turned into behaviour.
Main collaborators: Nina Lundholm, Niels Daugbjerg, Per Juel Hansen and Øjvind Moestrup (Uni. of Copenhagen) and Dan-E Nilsson and Mike Bok (Lund Uni.)
Sleep in sponges
Sleep is a somewhat enigmatic process, which is seen in most animals, at least if a broad definition is used. In present day animals this process serves two major functions: it is an inactive period of low metabolic rate saving energy for periods with high activity.
The inactivity also leads to less need for information processing by the nervous system, and the CNS goes through a number of important restructuring and repair processes during sleep. An interesting question is which of these two functions initially drove the evolution of sleep.
Sponges (Porifera) constitute one of the earliest branches in animal evolution and they lack a nervous system. We examine whether the diurnal rhythm found in some sponges includes a period of sleep, which would add strong support to energy saving being the original function of sleep.
Main collaborator: Peter Funch (Uni. of Århus)
Current courses: Organismernes Diversitet – Livets Træ (BSc level), Marine Biological summer course (BSc level), Sensory Biology (Developed course and course responsible, MSc level), Animal Morphology (MSc level) and Molecular Neurobiology (MSc level)
Previous courses: Experimental Marine Biology (BSc level), Marine Biology (MSc level), Applied Marine Biology (MSc level), Invertebrate Zoology (MSc level), and Evolutionary Biology (MSc level)
The projects listed below are all suitable for both bachelor and master projects.
Do organisms without a nervous system sleep?
Sleep is a somewhat enigmatic process, which is seen in most animals, but the evolutionary origin is unknown. So far sleep has only been tested in animals with a nervous system, but in this project you will test possible sleep in nerve-less animals and other organisms. There will be a focus on the basal animal group, sponges (Porifera), but the sister-group to all animals, choanoflagellates, will also be examined. The sponges are less active at night (https://doi.org/10.1242/jeb.244751) and using advanced microscopy and behavioural experiments on both whole sponges and so-called sandwich preparations you will test if this qualifies as sleep. If sponges sleep it will strongly suggest that sleep arose before neurons and that the original function was energy conservation and not as a period for nervous system repairs.
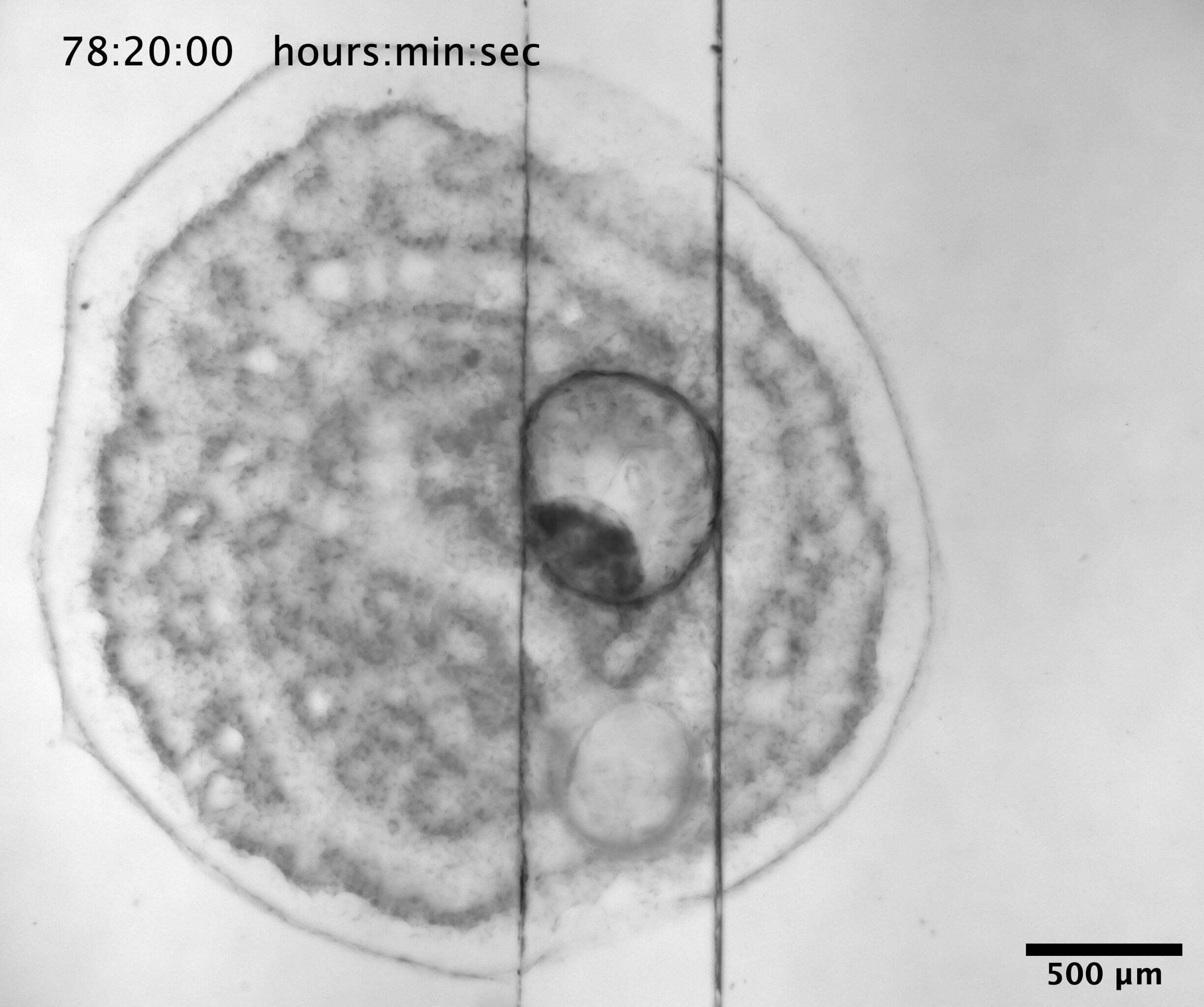
Vision in a single cell
A special group of single celled organisms, the dinoflagellate family Warnowiaceae, have a cell organelle (the ocelloid) resembling an eye. In this project you will examine the functional significance of this organelle with a focus on prey capture. The dinoflagellates shall be collected several places in the inner Danish Waters and tested in number of experiments in the laboratory. In a special setup their swim patterns can be video filmed while presenting them with images of naturally sized prey items. This will reveal if they can see images and if so also what type of information they respond to. Furthermore, some of the collected cells will be fixed for a microscopy, incl. electron and confocal microscopy, in order to examine the detailed structures of the ocelloid.
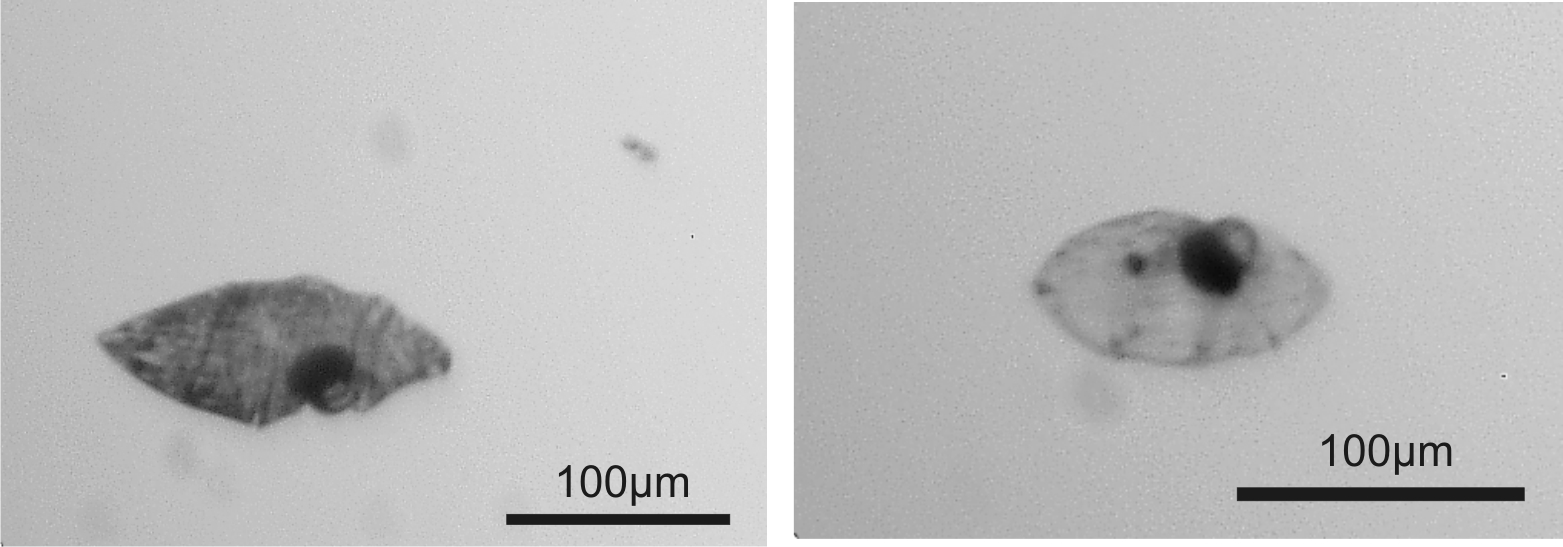
Vision in juvenile starfish
Most starfish, incl. the common Danish species Asterias rubens, have a compound eye at the tip of each arm. A compound eye is build of many separate optical units, ommatidia, each adding one pixel to the image, and adult A. rubens have approx. 150 ommatidia in each eye. The eye is formed when their larvae metamorphose into the juvenile starfish and interestingly they start out with only 4-5 ommatidia in each eye. The functional significance of this juvenile eye is unknown and the project will examine this through behavioural experiments and morphological examinations. You will collect live metamorphosing larvae in Øresund and Kattegat and perform phototaxis experiments in the lab. Furthermore, some of the specimens will be fixed for light, electron and confocal microscopy used to map the visual field and spatial resolution of the eye.

If any of these projects sounds interesting to you, you can contact me via e-mail: algarm@bio.ku.dk
|
Post Docs |
|
|
Ronald Petie |
2014-2016 |
|
Jan Bielecki |
2015-2016 |
|
Andreas Alterburger |
2019-2020 |
| Maria Portela (shared) |
2021-2022 |
| Alison Ruth Irwin |
2023-2025 |
|
PhD |
|
|
Megan O'Connor |
2005-2009 |
|
Ronald Petie |
2007-2012 |
|
Jan Bielecki |
2010-2013 |
|
Sofie Dam Nielsen |
2016-2021 |
|
Master students |
|
|
Mathieu Boudes |
2005 |
|
Peter Jonsson |
2005 |
|
Elin Isberg |
2006 |
|
Johanna Lindblom |
2007 |
|
Pär Söderquist |
2008 |
|
Robert Gad |
2008 |
|
Lisbet Lauridsen |
2011 |
|
Louise Würtz |
2012 |
|
Marion Lebouvier |
2014 |
|
Jussi Nygren |
2014 |
|
Louise Frandsen |
2015 |
|
Marianne Juhl |
2016 |
|
Mathias Guldberg Pedersen |
2016 |
|
Mia Hyldahl |
2016 |
|
Sabrina Beer |
2016 |
|
Sebastian Stamatis |
2017 |
|
Marie Helene Birk |
2017 |
|
Lene Hartmann Jensen |
2017 |
|
Majken Seier Islin |
2018 |
|
Ari Jespersen |
2018 |
|
Paula Gonzalez |
2019 |
|
Sofus Wiisbye |
2019 |
|
Sandra Helmark Hansen |
2019 |
|
David Kulcsar |
2020 |
|
Camilla Korsvig Nielsen |
2020 |
|
Sidsel Hald Simonsen |
2020 |
|
Nathalie Jacomo |
2020 |
|
Ditte Sundberg |
2020 |
|
Jens-Erik Sværke |
2021 |
|
Sarah Flensborg |
2021 |
|
Oliver Hamilton |
2021 |
|
Jakob Østergaard |
2022 |
|
Sarah Nørregaard Jensen |
2022 |
|
Alex Jn-Charles |
2022 |
|
Emma Probst |
2022 |
|
Frederik Fischer |
2022 |
| Laura Ferreira |
2023 |
| Caroline Risager Hansen |
2024 |
| Fabio Celestino |
2024 |
| Natasha Hansen |
2024 |
| Morten Pedersen |
2024 |
| Minik Rossing |
2024 |
| Kathrine Koch Jonsson |
2025 |
| Victoria Schiller-Steffansson |
2025 |
|
Bachelor students |
|
|
Simone Mori |
2008 |
|
Marie Helene Birk |
2014 |
|
Marianne Rrisager Kjøller |
2014 |
|
Mathilde Sort |
2014 |
|
Christina Fjorbak |
2014 |
|
Anne Majgaard Jensen |
2015 |
|
Camilla Korsvig-Nielsen |
2017 |
|
Sandra Helmark Hansen |
2017 |
|
Sofus Wiisbye |
2017 |
|
Freja Lauritsen |
2018 |
|
Nathalie Jacomo, |
2018 |
|
Tristan Leo Rintoull |
2018 |
|
Jessica Ceglarek |
2019 |
|
Signe Fraizer |
2019 |
|
Ida Jagd |
2019 |
|
Anton Søvad, |
2019 |
|
Emil Winkel |
2020 |
|
Line Lindholm |
2020 |
|
Line Kundby Frederiksen |
2020 |
|
Josephine Andresen |
2020 |
|
Jacob Bartholin |
2021 |
|
Julius Friss Petersen |
2021 |
|
Morten Bartholin |
2021 |
|
Lukas Kragh |
2022 |
| Pernille Kildebo-Jensen |
2022 |
| Caroline Assum |
2022 |
| Rasmus Kjær Kock |
2022 |
| Maria Bakhi |
2022 |
| Eva Sachaldemose |
2023 |
| Antonia Westfallen |
2023 |
| Emil Wirz |
2024 |
| Tobias Nielsen |
2024 |
| Nicoline Dolmer Skov |
2024 |
| Ellen Nielsen |
2025 |
| Sebastian Larsen |
2025 |
| Gregor Wöhle |
2025 |
Video 1
Video 2
Videos (1 and 2) from behavioural experiments on the visual ecology of the crown-of-thorns starfish. These experiments from a behavioural arena verify that the starfish use vision to seek out large dark structures in their habitat (coral boulders). Their blurry (low spatial resolution) vision does not allow them to detect the small dark spot and they walk randomly in the arena – the large circle is detected and they walk straight to it.
Video 3
Example of visually guided obstacle avoidance in the box jellyfish, Tripedalia cystophora. When seeing the obstacle (grey stripes on the wall) they turn 120-180 degrees in 2-4 rapid swim pulses. We use this behavior to test the visual system in box jellyfish incl. visually induced learning.
Ubegribeligt med Huxi Bach om blæksprutter
True Facts: Sea Stars - YouTube video
Podcast about the senses on Spotify
Vildt Naturligt om blæksprutter
Staff at Garm Lab
| Name | Title | Phone | |
|---|---|---|---|
| Anders Lydik Garm | Associate Professor | +4551827004 | algarm@bio.ku.dk |
| Constance Cubris | Postdoc | constance.cubris@bio.ku.dk | |
| Estelle Moubarak | Postdoc | estelle.moubarak@bio.ku.dk | |
| Mikala Bording | Master student | fkl635@alumni.ku.dk | |
| Shani Assouline | Master student | shani.assouline@sund.ku.dk | |
| Amalie Kargaard Jensen | Master student | amje@snm.ku.dk |
Picture gallery
 |
 |
| Eye of starfish Acanthaster planci. | Gill and potential nose of horseshoe crab. |
 |
 |
| The common Danish jellyfish Aurelia. aurita | Immunostain of nervous system in box jellyfish rhopalium. |
 |
 |
| The box jellyfish Tripedalia cystophora (courtesy J Bielecki). | The deadly box jellyfish Chironex fleckeri. |
Contact
Associate Professor
Anders Lydik Garm
Marine Biology Section
Bio Aqua, Room 20-1-116, 1st floor
DK-2100 Copenhagen Ø
algarm@bio.ku.dk
Phone: +45 51 82 70 04
ORCID: 0000-0002-2080-735X
News
3-Year postdoc position in Ctenophore bioluminescence, neurobiology and ecology (application deadline 1 March)
Anders i Det Billige Skidt med Alexander Holm og Sebastian Klein om sanser
Anders i Det Billige Skidt med Alexander Holm og Sebastian Klein om dinoflagellater
DFF2-projekt om lysende dræbergopler
Associative learning in the box jellyfish: Anders Garm gave a talk for the Levin Lab
Gopler Lærer Forlæns: Anders Garm svarer på Lone Franks 24 spørgsmål
Villum Experiment grant: Do animals glow with invisible light?
True Facts: How Jellyfish Hunt (Ze Frank YouTube video)
Alciopid worms in the news:
Exploring bioluminescence of deep-sea starfish with OceanX (YouTube)
Jellyfish-learning in the media: