The pH group
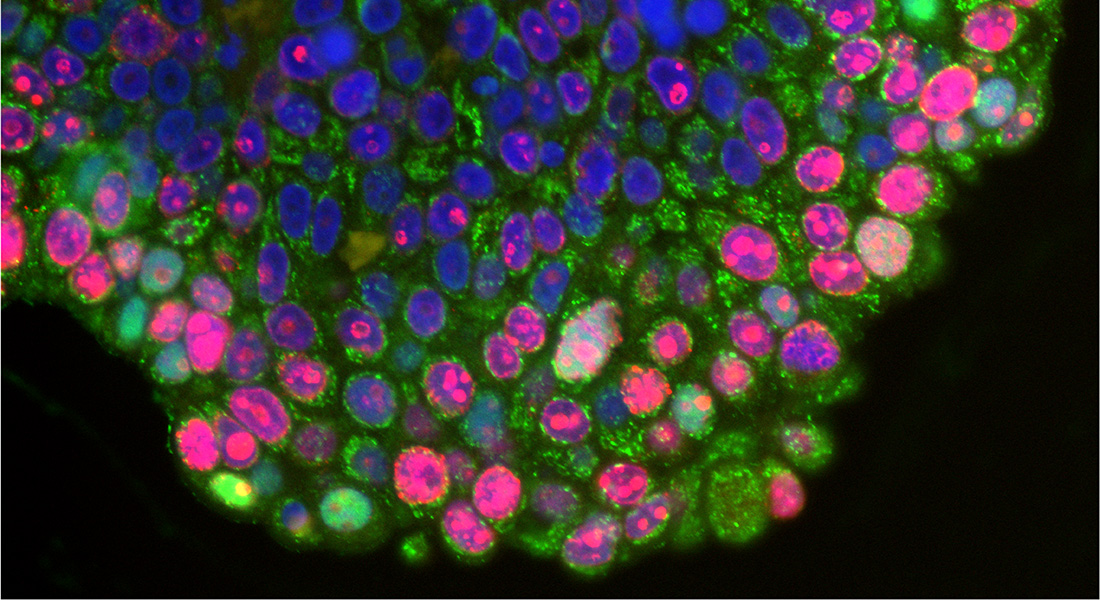
We study cellular acid-base homeostasis and associated signaling in normal physiology, cancer, and metabolic disease. Our specific interests are how pH is regulated in each cellular compartment, how it is sensed by cells, and how it in turn regulates cellular functions – as well as structure-function and regulatory interactions of pH regulatory transport proteins.
The key research interest of the pH group is how acid-base transporters are regulated and how their function and dysfunction impact human health. Our primary focus is the biology of SLC9A family Na+/H+ exchangers and SLC4A family Na+,HCO3- cotransporters. We are highly collaborative, and we employ a broad range of approaches from biophysics, genetics and bioinformatics, over cell- and molecular biology to animal studies, to understand the roles of acid/base transporters in health and disease. In the past years, we have provided important insights into how these transporters are regulated in cancer and contribute to its progression and metabolism. Currently, we are expanding this to include, on the one hand, other metabolites than protons in cancer, and on the other hand, the roles of acid-base dysregulation in other diseases than cancer, including metabolic liver diseases and neurodegeneration.
The bulk of our projects are devoted to understanding how the acidic tumor microenvironment regulates cancer progression. This includes the roles of acid-base homeostasis in epithelial cancer development, which we study using stem cell based organoids from transgenic pancreas cancer mouse models as well as in human cancer cells; the involvement of specific acid-base transporters in shaping cancer cell phenotype; and the mechanisms through which cytosolic, organellar, and extracellular pH regulate cellular signaling and how this is altered in cancer cells.
- However, in recent years our interest have expanded to understanding, on the one hand, the roles of other metabolites than acid in cancer; and on the other hand, the roles of acid-base disturbances in other diseases than cancer. Thus, our current projects also include:
- Development of a microfluidics-based system combined with spatial transcriptomics, to precisely control pH, lactate, and oxygen gradients in time and space and study their impact on cancer- and stromal cell behavior in the tumor microenvironment.
- Addressing the role of NHE1 in non-alcoholic steatohepatitis (NASH), using both in vitro systems and a liver-specific NHE1 knockout mouse
- Studying the mechanisms through which point mutations in the endosome-specific, X chromosome-expressed NHE isoform NHE6 cause devastating neurodegenerative disease in boys
- Investigating how the lactate receptor HCAR1 (GPR81) contributes to cancer development by altering recruitment of anti-cancer immune cells to tumors
We have ongoing collaborations with several groups both in Denmark and abroad, including:
Prof. Albin Sandelin, BIO
Prof. Henriette Pilegaard, BIO
Prof. Birthe Kragelund, BIO
Prof. Thue Schwartz, Novo Nordisk Center for Basic Metabolism, UCPH Faculty of Health
Dr. Kenji Maeda and Dr. Bin Liu, Danish Cancer Society Research Center
Dr. Rodolphe Marie, Technical University of Denmark
Prof. Dr. Luis A. Pardo, Max Planck Institute for Experimental Medicine, Göttingen, DE
Dr. Eric Morrow, Brown University, Rhode Island, USA
Dr. Dario Longo, University of Torino, Italy
And last but not least, the pHIoniC ITN: https://www.medizin.uni-muenster.de/phionic/home.html
2023
Swietach P, Boedtkjer E, Pedersen SF. How protons pave the way to aggressive cancers. Nat Rev Cancer. 2023 Oct 26. doi: 10.1038/s41568-023-00628-9. Epub ahead of print. PMID: 37884609.
Czaplinska D, Ialchina R, Andersen HB, Yao J, Stigliani A, Dannesboe J, Flinck M, Chen X, Mitrega J, Gnosa SP, Dmytriyeva O, Alves F, Napp J, Sandelin A, Pedersen SF. Crosstalk between tumor acidosis, p53 and extracellular matrix regulates pancreatic cancer aggressiveness. Int J Cancer. 2023 Mar 15;152(6):1210-1225. doi: 10.1002/ijc.34367.
Rolver MG, Holland LKK, Ponniah M, Prasad NS, Yao J, Schnipper J, Kramer S, Elingaard-Larsen L, Pedraz-Cuesta E, Liu B, Pardo LA, Maeda K, Sandelin A, Pedersen SF. Chronic acidosis rewires cancer cell metabolism through PPARα signaling. Int J Cancer. 2023;152(8):1668-1684. doi: 10.1002/ijc.34404
Severin M, Pedersen EL, Borre MT, Axholm I, Christiansen FB, Ponniah M, Czaplinska D, Larsen T, Pardo LA, Pedersen SF. Dynamic localization of the Na+-HCO3- cotransporter NBCn1 to plasma membrane, centrosomes, spindle, and primary cilia. J Cell Sci. 2023 Apr 1;136(7):jcs260687. doi: 10.1242/jcs.260687
Edamana S, Pedersen SF, Nejsum LN. Aquaporin water channels affect the response of conventional anticancer therapies of 3D grown breast cancer cells. Biochem Biophys Res Commun. 2023 Jan 8;639:126-133. doi: 10.1016/j.bbrc.2022.11.096
Panou DA, Pedersen SF, Kristensen M and Nielsen HM (2023), Epithelium dynamics differ in time and space when exposed to the permeation enhancers penetramax and EGTA. A head-to-head mechanistic comparison.Front. Drug Deliv. 3:1221628. doi: 10.3389/fddev.2023.1221628
Lundø K, Dmytriyeva O, Spøhr L, Goncalves-Alves E, Yao J, Blasco LP, Trauelsen M, Ponniah M, Severin M, Sandelin A, Kveiborg M, Schwartz TW, Pedersen SF. Lactate receptor GPR81 drives breast cancer growth and invasiveness through regulation of ECM properties and Notch ligand DLL4. BMC Cancer, in press
2022
Elingaard-Larsen L, Rolver MG, Sørensen EE, Pedersen SF. How reciprocal interactions between the tumor microenvironment and ion transport proteins drive cancer progression. Rev Physiol Biochem Pharmacol. 2022;182:1-38. PMID: 32737753
Schnipper J, Kouba S, Hague F, Girault A, Rybarczyk P, Telliez MS, Guénin S, Tebbakha R, Sevestre H, Ahidouch A, Pedersen SF, Ouadid-Ahidouch H. The TRPC1 Channel Forms a PI3K/CaM Complex and Regulates Pancreatic Ductal Adenocarcinoma Cell Proliferation in a Ca2+-Independent Manner. Int J Mol Sci. 2022;23(14):7923. PMID: 35887266
Schnipper J, Kouba S, Hague F, Girault A, Rybarczyk P, Telliez MS, Guénin S, Tebbakha R, Sevestre H, Ahidouch A, Pedersen SF, Ouadid-Ahidouch H. The TRPC1 Channel Forms a PI3K/CaM Complex and Regulates Pancreatic Ductal Adenocarcinoma Cell Proliferation in a Ca2+-Independent Manner. Int J Mol Sci. 2022;23(14):7923. doi: 10.3390/ijms23147923. PMID: 35887266
2021
Pedersen SF, Flinck M, Pardo LA. The Interplay between Dysregulated Ion Transport and Mitochondrial Architecture as a Dangerous Liaison in Cancer. Int J. Mol. Sci. 2021; 22(10):5209. PMID: 34069047 (featured on front page)
Andersen, H.B., Ialchina, R., Pedersen, S.F., Czaplinska, D. Metabolic reprogramming by driver mutation-tumor microenvironment interplay in pancreatic cancer: new therapeutic targets. Cancer Metastasis Rev2021;40(4):1093-1114. PMID: 34855109
Sjøgaard-Frich LM, Prestel A, Pedersen ES, Severin M, Kristensen KK, Olsen JG, Kragelund BB, Pedersen SF. Dynamic Na+/H+ Exchanger 1 (NHE1):Calmodulin complexes of varying stoichiometry and structure regulate Ca2+-dependent NHE1 activation. Elife. 2021;10:e60889. doi: 10.7554/eLife.60889. PMID: 33655882.
Doray A, Lemoine R, Severin M, Chadet S, Lopez-Charcas O, Héraud A, Baron C, Besson P, Monteil A, Pedersen SF, Roger S. The Voltage-Gated Sodium Channel Beta4 Subunit Maintains Epithelial Phenotype in Mammary Cells. 2021;10(7):1624. PMID: 34209614
2020
Rolver MG, O. Elingaard-Larsen L, Counillon L, Pedersen SF. 2020. Pyrazine ring-based Na+/H+ exchanger (NHE) inhibitors potently inhibit cancer cell growth in 3D culture, independent of NHE1. Sci Rep 10(1):5800. PMID: 32242030
Cholak E, Bugge K, Khondker A, Gauger K, Pedraz-Cuesta E, Enghave Pedersen M, Bucciarelli S, Vestergaard B, Pedersen SF, Rheinstädter M, Langkilde AE, Kragelund BB. Avidity within the N-terminal anchor drives α-synuclein membrane interaction and insertion. FASEB J. 2020 Jun;34(6):7462-7482. PMID: 32277854
Flinck M, Hagelund SE, Gorbatenko A, Severin M, Pedraz-Cuesta E, Novak I, Stock CM, Pedersen SF. The Vacuolar H+ ATPase α3 Subunit Negatively Regulates Migration and Invasion of Human Pancreatic Ductal Adenocarcinoma Cells. Cells. 2020;9(2):465. PMID: 32085585
Bagdonaite I, Pallesen I, Ye Z, Vakhrushev SY, Marinova I, Nielsen M, Kramer S, Pedersen SF, Joshi HJ, Bennett EP, Dabelsteen S, Wandall H. O-glycan initiation directs distinct biological pathways and controls epithelial differentiation. EMBO Reports 2020;21(6):e48885. PMID: 32329196
Yao J, Czaplinska D, Ialchina R, Schnipper J, Liu B, Sandelin A*, Pedersen SF*. Cancer Cell Acid Adaptation Gene Expression Response Is Correlated to Tumor-Specific Tissue Expression Profiles and Patient Survival. Cancers (Basel). 2020;12(8):2183. PMID: 32764426 (*co-corresponding)
Malinda RR, Zeeberg K, Sharku PC, Ludwig MQ, Pedersen LB, Christensen ST, Pedersen SF. TGFβ Signaling Increases Net Acid Extrusion, Proliferation and Invasion in Panc-1 Pancreatic Cancer Cells: SMAD4 Dependence and Link to Merlin/NF2 Signaling. Front Oncol. 2020;10:687. eCollection 2020. PMID: 32457840
Hendus-Altenburger R, Vogensen J, Pedersen ES, Luchini A, Araya-Secchi R, Bendsoe AH, Prasad NS, Prestel A, Cardenas M, Pedraz-Cuesta E, Arleth L, Pedersen SF, Kragelund BB. The intracellular lipid-binding domain of human Na+/H+ exchanger 1 forms a lipid-protein co-structure essential for activity. Commun Biol. 2020;3(1):731. doi: 10.1038/s42003-020-01455-6. PMID: 33273619;
Lundø K, Trauelsen M, Pedersen SF*, Schwartz TW*. Why Warburg works: Lactate controls immune evasion through GPR81. Cell Metabolism 2020, ;31(4):666-668, PMID: 32268113
Elingaard-Larsen L, Rolver MG, Sørensen EE, Pedersen SF. How reciprocal interactions between the tumor microenvironment and ion transport proteins drive cancer progression. Rev Physiol Biochem Pharmacol. 2020 Aug 1. doi: 10.1007/112_2020_23. Online ahead of print. PMID: 32737753
Rolver MG, Pedersen SF. Putting Warburg to work: how imaging of tumour acidosis could help predict metastatic potential in breast cancer. Br J Cancer. 2020. doi: 10.1038/s41416-020-01171-2. Epub ahead of print. PMID: 33257840
Boedtkjer E and Pedersen SF. 2020. The acidic tumor microenvironment as a driver of cancer. Annual Rev Physiol 82:103-126. PMID: 31730395
2019
Jensen HH, Pedersen GA, Morgen JJ, Parsons M, Pedersen SF, Nejsum LN. The Na+/H+ exchanger NHE1 localizes as clusters to cryptic lamellipodia and accelerates collective epithelial cell migration. J Physiol. 2019, 597(3):849-867. PMID: 30471113
Gorbatenko A, Søkilde R, Sorensen EE, Newie I, Persson H, Morancho B, Arribas J, Litman T, Rovira C, Pedersen SF. 2019. HER2 and p95HER2 differentially regulate miRNA expression in MCF-7 breast cancer cells and downregulate MYB proteins through miR-221/222 and miR-503. Sci Rep. 2019, 9(1):3352. PMID: 30833639
Bialik JF, Ding M, Speight P, Dan Q, Miranda MZ, Di Ciano-Oliveira C, Kofler MM, Rotstein OD, Pedersen SF, Szászi K, Kapus A. Profibrotic epithelial phenotype: a central role for MRTF and TAZ. Sci Rep. 2019, 9(1):4323. PMID: 30867502
Rolver MG, Elingaard-Larsen LO, Pedersen SF. Assessing Cell Viability and Death in 3D Spheroid Cultures of Cancer Cells. J Vis Exp 2019, 148. PMID: 31259899
Hendus-Altenburger R, Wang X, Sjøgaard-Frich L, Pedraz-Cuesta E, Sheftic SR, Bendsøe AH, Page R, Kragelund BB, Pedersen SF, Peti W. 2019. Molecular basis for the binding and selective dephosphorylation of Na+/H+ exchanger 1 by calcineurin. Nat Comm 10(1):3489. PMID: 31375679
Tornabene E, Helms HCC, Pedersen SF, Brodin B. 2019. Effects of oxygen-glucose deprivation (OGD) on barrier properties and mRNA transcript levels of selected marker proteins in brain endothelial cells/astrocyte co-cultures. PLoS One;14(8):e0221103. eCollection 2019. PMID: 31425564
Pedersen SF and Counillon L. 2019. The SLC9A-C mammalian Na+/H+ exchanger family: Molecules, mechanisms, and physiology. Physiol Rev 99 (4), 2015-2113, PMID: 31507243
Czaplinska D, O. Elingaard-Larsen L, Rolver MG, Severin M, Pedersen SF. 2019. 3D multicellular models to study the regulation and roles of acid-base transporters in breast cancer. Biochem. Soc. Trans. 47 (6), 1689-1700, PMID: 31803922
2018
Andersen AP, Samsoe-Petersen J, Oernboe EK, Boedtkjer E, Moreira JMA, Kveiborg K, Pedersen SF. The net acid extruders NHE1, NBCn1 and MCT4 promote mammary tumor growth through distinct but overlapping mechanisms. Int J Cancer 2018 142(12):2529-2542. PMID: 29363134
Olesen CW, Vogensen J, Pedersen IS, Stemann JHV, Schröder JM, Christensen DP, Pedersen SF. Trafficking, localization and degradation of the Na+,HCO3– cotransporter NBCn1 in kidney and breast epithelial cells. Sci Rep 2018, 8(1):7435. PMID: 29743600
Flinck M, Kramer SH, Schnipper J, Andersen AP, Pedersen SF. The acid-base transport proteins NHE1 and NBCn1 regulate cell cycle progression in human breast cancer cells. Cell Cycle 2018, 17(9):1056-1067. PMID: 29895196
Lee S, Axelsen TV, Jessen N, Pedersen SF, Vahl P, Boedtkjer E. Na+,HCO3–-cotransporter NBCn1 (Slc4a7) accelerates ErbB2-induced breast cancer development and tumor growth in mice. Oncogene, 2018, 37(41):5569-5584. PMID: 29907770
Flinck M, Kramer SH, Pedersen SF. 2018. Roles of pH in control of proliferation. Acta Physiol (Oxf), 223(3):e13068. PMID:29575508
We contribute to the following courses in the Department of Biology:
General Cell Biology (Almen Cellebiologi)
Signal Transduction (Signaltransduktion)
Advanced Cell Biology
Developmental Biology
Cellular and Integrative Biology
Cellular Signaling in Health and Disease
The Carlsberg Foundation
The Danish Cancer Society
H2020-MSCA-ITN-2018 813834
NNF - Endocrinology project
NNF - Interdisciplinary Synergy project
Independent Research Fund Denmark – Medical Sciences
Independent Research Fund Denmark – Natural Sciences
Lectures
UCPH Forward: Lectures on motivation and role models in research (2019, 2020)
TALENT.DK: Seminar 2020 (https://www.talent-dk.dk/viden-arkiv/materiale-fra-seminarer/2020-seminar/)
Podcast
Science Stories (https://den2radio.dk/udsendelser/science-stories-luca/ and on Spotify/iTunes)
Book
“Fortællinger fra Grundforskningens Grænseland”, published 2020 by Royal Danish Academy of Science and Letters and the Danish National Research Foundation (https://dg.dk/en/new-book-on-basic-research-from-the-danish-national-research-foundation/).

Not least, we have a lot of fun!
Some highlights:
Life in the lab

Cup painting November 2023
Chilling with Nobel Prize winner Prof. William Kaelin in the Black Diamond, Sept. 2023

Chinese dinner, Feb. 2023

Group members
| Stine Falsig Pedersen | Professor | sfpedersen@bio.ku.dk |
| Marc Severin | PhD student | marc.severin@bio.ku.dk |
| Lise Sjøgaard-Frich | PhD student | lise.sjoegaard@bio.ku.dk |
| Lara Kristin Sach | PhD student | lara.kristin.sach@sund.ku.dk |
| Eliana Carolina Goncalves Alves | Postdoc | eliana.alves@bio.ku.dk |
| Leïla Dos Santos | Postdoc | leila.dossantos@bio.ku.dk |
| Michala Gylling Rolver | Postdoc (50%) | michala.rolver@bio.ku.dk |
| Mohamed Chamlali | Postdoc (50%) | mohamed.chamlali@bio.ku.dk |
| Mett Flinck | Lab manager | mette.flinck@bio.ku.dk |
| Tanja Larsen | Technician | tanja.larsen@bio.ku.dk |
| Frida Jolande Birkbak | Student assistant | frida.birkbak@bio.ku.dk |
| Sofie Cens Holste | Master's student | hxw600@alumni.ku.dk |
| Julie Hindkær | Master's student | gxb865@alumni.ku.dk |
| Rikke Kjer Hansen | Master's student | dts138@alumni.ku.dk |
| Philipp Alexander Rüdiger Bauer | Master's student | kxc357@alumni.ku.dk |
Contact
Professor
Stine Helene Falsig Pedersen
Cell Biology and Physiology
Universitetsparken 13
DK-2100 Copenhagen Ø, Denmark
sfpedersen@bio.ku.dk
Phone: +45 35 32 15 46
Mobile: +45 20 99 15 55
ORCID: 0000-0002-3044-7714
